User login
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
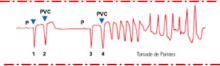
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
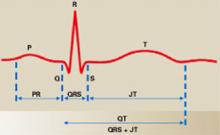
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
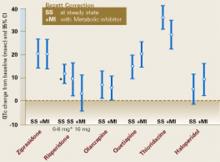
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
Before approving the antipsychotic agent ziprasidone last year, the Food and Drug Administration required specific safety data on whether the drug might cause the life-threatening arrhythmia known as torsade de pointes.
The FDA’s action, which delayed the drug’s approval for 3 years, underscores growing concern about the risk of cardiovascular effects with the use of antipsychotic and other agents known to prolong the cardiac QT interval. This concern has led to withdrawal of some drugs before reaching the market (e.g., the atypical neuroleptic sertindole), the addition of “black box” warnings in the labeling of some antipsychotics, and withdrawal from the market of antihistamines terfenadine and astemizole and the GI stimulant cisapride.
Torsade de pointes is a polymorphic ventricular tachycardia (VT), a rare arrhythmia that can cause sudden death. Because torsade can occur with the use of some antipsychotics, the psychiatrist needs to consider cardiovascular safety when selecting among available agents. To help with these decisions, here is information about the documented and potential electrocardiographic features of commonly prescribed antipsychotic drugs, as well as background on QT interval prolongation and torsade de pointes.
Torsade de pointes
Named for a ballet movement, torsade de pointes describes bursts of “twisting of the points,” a variation of the morphology of the QRS vector about the isoelectric axis from positive to net negative and back again. As seen on an ECG (Figure 1), the first beat of torsade de pointes is a normal ventricular complex preceded by a P wave. This is followed by a premature ventricular contraction (PVC) with a short coupling interval. After a compensatory pause, a second normal beat is followed by a second PVC, which is the first beat of a polymorphic VT. We know tachycardia is present because the ventricular beats appear close together. We know the arrhythmia is ventricular in origin because the ventricular complexes are wide. Finally, we note the ventricular complexes vary in configuration—that is, the shape (morphology) varies from beat to beat.
Figure 1 Typical ECG features of torsade de pointes
Sinus beat with normal ventricular complex (1) followed by premature ventricular contraction (PVC) (2) with short coupling interval. After a long pause (long refractory period), another sinus beat (3) is followed by another PVC (4) with a short coupling interval. The second PVC (4) is the first beat of polymorphic ventricular tachycardiaIn torsade, the stimulus for the VT moves within the ventricle, changing its shape from beat to beat. This multifocal VT differs from the more common unifocal VT, in which all the QRS complexes appear the same.
Drug-induced torsade de pointes
Although the term torsade de pointes was first described in 1966,1 the drug-induced form of this arrhythmia has been recognized for nearly a century.
Quinidine Around 1920, cardiologists first used quinidine to help restore normal sinus rhythm in patients with atrial fibrillation, most commonly due to rheumatic heart disease.2
In 1964, Selzer and Wray3 studied the use of quinidine to convert atrial fibrillation to normal sinus rhythm in more than 200 patients seen during 4 years in a cardiopulmonary clinic. In a subgroup of eight patients, these researchers documented 10 reactions (including five documented episodes of ventricular fibrillation/ventricular flutter) among 36 syncopal episodes that developed within 1 to 6.5 hours of quinidine administration. Symptoms were nonspecific and included nausea, faintness, and feeling ill. It is now recognized that torsade de pointes was the principal rhythm disturbance in those eight patients. Syncope usually occurs early in treatment and may be found in 5% to 10% of patients taking quinidine.
TCAs and antipsychotics Tricyclic antidepressants (TCAs) and antipsychotics that have quinidine-like properties (e.g., thioridazine) also may be associated with QT interval prolongation and torsade de pointes.4-9 In high doses (particularly in overdose), TCAs may induce widening of the QRS complex. Fowler et al reported episodes of VT in five patients taking thioridazine—one of whom died.10
Mehtonen et al reported sudden unexpected deaths associated with antipsychotic or antidepressant drugs among 31 women and 18 men in a survey of autopsies performed from 1985 to 1988 in Finland. The authors documented therapeutic use of phenothiazines in all but 3 of the 49 cases. Thioridazine was involved in more than half the deaths. In 15 of the deaths, thioridazine was the only antipsychotic drug taken. Drugs other than thioridazine were documented in only 5 of the 49 sudden cardiac deaths.11
Figure 2 Normal ECG in sinus rhythm
In this typical lead II of a surface ECG, the P wave (atrial depolarization) leads to right and left atrial contraction and the QRS complex (ventricular depolarization) leads to left and right ventricular contraction. The ST segment represents isoelectric ventricular repolarization, and the T wave represents directional repolarization. The QT interval includes both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval, or ST segment plus T wave).
QT interval as a marker for torsade
The incidence of torsade is unknown, but it is an uncommon cardiac abnormality. In the United States, torsade probably accounts for less than 5% of the 300,000 sudden cardiac deaths that occur each year. Because torsade de pointes is rare, regulatory agencies and clinicians use the QT interval as a surrogate ECG marker for risk of torsade de pointes. Heart rate can affect the QT interval, so various formulae are used to correct the QT interval for heart rate (QTc).
What is the QT interval? In a normal ECG (Figure 2), the P wave derives from right and left atrial electrical depolarization. The pacemaker of the heart is located in the sino-atrial node (SAN) in the superior portion of the right atrium. From the SAN, electrical signals travel down three intra-atrial pathways, activating the right atrium, then travel to the atrioventricular node (AVN). Bachmann’s bundle—a fourth atrial pathway—passes from the SAN to depolarize the left atrium. From the AVN, the electrical signal travels through the left and right bundle branches to activate their respective ventricles.
Electrical depolarization of the left and right ventricles produces the QRS complex. Most of the electrical forces making up this complex arise in the left ventricle, which is much larger than the right ventricle.
The electrical circuitry of the heart activates the left and right atria in such a fashion that these chambers eject blood into their respective ventricles just before these chambers contract. Optimal ventricular filling maximizes ventricular ejection of blood (Starling’s law). Ventricular repolarization (JT interval—electrical recovery) follows ventricular depolarization. On the surface ECG, the JT interval consists of an isoelectric event—the ST segment running from the end of the QRS complex to the beginning of the T wave—and the T wave itself (directional electrical recovery).
The QT interval, then, consists of both ventricular depolarization (QRS complex) and ventricular repolarization (JT interval). Ventricular repolarization makes up by far the greater portion of the QT interval.
Correcting the QT interval (QTc) In 1920, Bazett noted that as the heart rate slowed, the QT interval lengthened.12 From personal and reported observations, he derived an equation called the Bazett formula that corrects (or normalizes) the QT interval to a heart rate of 60 beats/min (QTc). In the Bazett formula, the QTc interval is the measured QT interval divided by the square root of the RR interval (time between sequential QRS complexes—the determinant of heart rate) measured in seconds (QTc = QT/RR).
The Bazett formula is most widely used to estimate the QTc interval, although at least 20 other formulae have been developed in response to the original’s perceived inadequacies.13-15 Bazett’s formula is used in most automated interpretations of the ECG.
Up to age 55, the normal QTc interval ranges from 350 to 430 msec for men and 350 to 450 msec for women, and it tends to increase with age. Most cases of torsade occur when the QT or QTc interval is greater than 500 msec.14 A QTc interval between 450 and 500 msec is cause for concern; a QTc interval that exceeds 500 msec is cause for alarm.
Factors that cause variations in QTc
Factors that can affect the QTc interval and increase the risk of torsade de pointes include electrolyte imbalances, medication use and overdose, cardiac disease, liver disease, endocrine disorders such as diabetes and hypothyroidism, and CNS injury (Table 1).
Table 1
Risk factors contributing to QTc interval prolongation
| Risk factor | Causes/implications |
|---|---|
| Sex (female) | QT intervals longer in women than in men QT interval longer during first half of menstrual cycle |
| Age (elderly) | Increased risk for CAD Multiple medications Pharmacokinetic/pharmacodynamic changes |
| Electrolyte imbalance Hypokalemia, hypomagnesemia Hypocalcemia | Diuretic use Excessive vomiting or diarrhea Postprandial hypokalemia |
| Congenital long QT syndrome | Associated with torsade and sudden death |
| Cardiac disease, with history of acute or chronic myocardial ischemia, CHF, cardiac arrhythmias, bradycardia | Increased risk of cardiac arrhythmias |
| Drugs known to prolong QTc interval | May potentiate QTc prolongation |
| Medication overdose with drugs that prolong the QTc interval | QTc prolongation generally dose-dependent |
| Concomitant medications, liver disease | Adverse events with cytochrome P-450 enzyme system inhibition, leading to increased drug levels that can increase QT interval |
| Endocrine/metabolic disorders Diabetes, obesity Hypothyroidism, pituitary insufficiency | Via electrolytes or cardiovascular disease |
| CNS injury Stroke, infection, trauma | Via autonomic nervous system dysfunction |
Circadian patterns The QTc interval varies throughout the 24-hour day, with nocturnal values about 20 msec greater than daytime measurements. These differences are driven by changes in autonomic (sympathetic and parasympathetic) tone.16,17 In 20 normal subjects, circadian variability was 76 ± 19 msec (range 35 to 108 msec) from day to night.17 This circadian variation may be accentuated in patients with cardiovascular disease.
Sex. At birth, QTc interval measurements do no vary by sex.18 At puberty, however, the male QTc interval shortens and remains shorter than its female counterpart by about 20 msec until age 50 to 55, coincident with a decline in male testosterone levels. This sex difference appears to be androgen driven. About 70% of torsade de pointes cases occur in women.18
Menstrual cycle QTc interval measurements are stable throughout the menstrual cycle if quinidine-like drugs are not given.
Variations were seen, however, when Rodriguez et al studied the effect of IV low-dose ibutilide (an antiarrhythmic agent known to prolong the QT interval) on the QTc intervals of 58 healthy subjects (38 men and 20 women, ages 21 to 40). During 1 month, men were studied once and women studied three times, coincident with the three phases of the menstrual cycle. The greatest increase in QTc intervals measurements occurred in women during the first half of their menstrual cycles.19
Age and cardiovascular disease Two congenital long QT syndromes may be associated with sudden death, mostly in children and young adults:
- The Jervell and Lange-Nielsen syndrome is marked by severe congenital deafness and autosomal recessive inheritance.
- The Romano-Ward syndrome has normal hearing and autosomal dominant inheritance.20
Congenital long QT syndrome (LQTS) occurs in about one in 5,000 births and accounts for about 3,000 to 4,000 deaths per year in the United States. Nine percent of pediatric LQTS subjects present with sudden cardiac death. More than 71% of patients will die before age 15 if not treated.
Elderly persons tend to have longer QTc intervals than do younger subjects, even when both groups are free of cardiovascular disease.21 Also, age-matched subjects with cardiovascular disease tend to have longer QTc intervals than do those free of cardiovascular disease.
Electrolytes Electrolyte disturbances, particularly hypokalemia and hypomagnesemia, may contribute to or even cause QT interval prolongation.22
Hypokalemia prolongs the cardiac action potential and may cause early afterdepolarization, leading to torsade.23 Low potassium levels reduce the net outward potassium current during phase 3 of the cardiac action potential. Hypomagnesemia may contribute to gross U wave alternans, lengthening the cardiac action potential and setting the stage for torsade.24 Various factors may contribute to electrolyte disturbances, including use of diuretics and excessive vomiting and diarrhea. Even postprandial states may induce hypokalemia.
Intensive exercise and agitation may be associated with hypokalemia.25 Serum potassium may be lower in severely agitated patients (3.59 mmol/L) than in mildly agitated patients (3.79 mmol/L). The mean QTc interval of psychiatric emergency patients may be prolonged (453±40 msec),5 with QTc intervals of psychiatric inpatients longer than those of psychiatric outpatients. Altered potassium states probably explain these observations. Mechanisms that link exercise and agitation with hypokalemia remain to be elucidated.
Metabolic factors Drugs may alter phase 3 potassium flow, thereby disrupting the synchrony of action of individual cardiac cells during repolarization. This change may induce early afterdepolarizations and torsade.23
Five percent to 10% of Americans of European descent have genetic profiles that make them poor metabolizers of drugs that are metabolized by the cytochrome P-450 isoenzyme 2D6. The Pfizer Inc. 054 study assessed the potential for metabolic inhibitors such as paroxetine to raise antipsychotic drug levels in these patients and induce QTc interval prolongation.26
In response to FDA concerns about QTc interval prolongation associated with the use of ziprasidone, Pfizer studied the potential for QTc interval prolongation when antipsychotics are given with and without metabolic inhibitors of cytochrome P-450 isoenzymes 2D6 (paroxetine), 3A4 (ketoconazole), and 1A2 (fluvoxamine). The study population of 183 subjects (mean age:men, 37.1 years, women 38.8 years) was three-quarters young men with schizophrenia, in good health otherwise and possessing normal ECGs—i.e., patients with a low risk of developing cardiac arrhythmias.
Figure 3 Antipsychotic drugs and QTc interval changes
Six antipsychotic drugs and QTc interval changes from baseline when given with and without metabolic inhibitors. QTc interval changes (in msec) when given without a metabolic inhibitor were ziprasidone, 20.3; risperidone, 11.6; olanzapine, 6.8; quetiapine, 14.5; thioridazine, 35.6; and haloperidol 4.7.
Reprinted from: “FDA Psychopharmacological Drugs Advisory Committee. 19 July 2000. Briefing Documents for Zeldox Capsules (Ziprasidone HCL). Pfizer.” Available from Central Research Division, Pfizer, Inc., Eastern Point Road, Groton, CT 06340, (860) 441-4100.Over the course of about 1 week, daily doses were escalated to ziprasidone, 160 mg; risperidone, 8 mg and 16 mg; olanzapine, 20 mg; quetiapine, 750 mg; thioridazine, 300 mg; and haloperidol, 15 mg. Thioridazine (35.6 msec) and ziprasidone (20.3 msec) showed the greatest QTc interval increase following drug administration (Figure 3). Co-administration of a metabolic inhibitor did not further prolong the QTc interval for these two drugs.
Of the six drugs studied, only thioridazine and ziprasidone showed QTc interval increases 5% compared with baseline measurements.
Co-administration of a metabolic inhibitor caused the greatest increase in QTc intervals for quetiapine (from 14.5 to 19.7 msec). This value closely approached the steady-state ziprasidone measurement (20.3 msec). Because quetiapine is more likely than the other antipsychotic drugs studied to increase heart rate, it may be argued that the Bazett formula’s limitations in estimating the QTc interval at higher heart rates contributed to the quetiapine study findings.
Table 2
Relative risk of QTc interval prolongation with common antipsychotic agents
| Risk level | Agent |
|---|---|
| ECG required or strongly recommended before prescribing (most commonly associated with QTc interval prolongation and torsade de pointes) | Thioridazine Mesoridazine Droperidol Pimozide Haloperidol in large doses IV (commonly ≥ 100 mg/d) |
| Mild to moderate risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Quetiapine Ziprasidone Chlorpromazine |
| Little or no risk of QTc interval prolongation (~20 msec) when prescribed alone or with a metabolic inhibitor | Haloperidol (oral) Olanzapine Risperidone Clozapine |
Recommendations
Taking a careful history is key to cardiovascular assessment before prescribing an antipsychotic. An ECG is indicated for patients with:
- Personal or family history of syncope or sudden death;
- Personal history of angina pectoris, myocardial infarction, congestive heart failure, cardiac arrhythmias, hypokalemia, hypomagnesemia, or significant cardiac risk factors.
The relative cardiovascular risks associated with antipsychotic agents are shown in Table 2.
An ECG also is required or strongly recommended before prescribing the antipsychotic drugs most commonly associated with QT prolongation and torsade de pointes—droperidol, haloperidol in large doses IV (commonly 100 mg/d), mesoridazine, pimozide, and thioridazine.
The FDA has strengthened the warning labels required for these agents, adding “black box” warnings about the risks of prolonged QTc intervals, torsade de pointes, and sudden death for droperidol, mesoridazine, and thioridazine. Thioridazine, for example, is indicated only for patients with schizophrenia who fail to show an acceptable response to other antipsychotic drugs. Its use is contraindicated in patients who take:
- fluvoxamine, propranolol, and pindolol;
- any drug that inhibits the cytochrome P-450 2D6 isoenzyme (e.g., fluoxetine, paroxetine);
- agents known to prolong the QTc interval.
Use of thioridazine also is contraindicated in patients known to have reduced levels of the cytochrome P450 2D6 isozyme, as well as in patients with congenital LQTS or a history of cardiac arrhythmias. Psychiatrists are advised to read the warnings and prescribing information in the labeling of all antipsychotics for potential cardiovascular side effects.
When the psychiatrist receives a report of suspected QTc interval prolongation on a patient’s ECG, the following steps are recommended:
- Obtain another ECG.
- Assess serum potassium, magnesium, calcium, and thyroid hormone levels.
In patients with confirmed QTc interval prolongation, any complaint of palpitations, presyncope, or syncope are grounds for urgent referral to a cardiologist.
Related resources
- European Society of Cardiology guidelines: www.escardio.org/scinfo/Guidelines/Haverkamp.pdf
- Sudden Arrhythmia Death Syndromes Foundation (SADSF): www.sads.org (800) 786-7723.
- Drugs that prolong the QT interval and/or induce torsade de pointes. Georgetown Center for Education and Research Therapeutics: www.torsades.org
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.
1. Dessertenne F. Tachycardie ventriculaire a deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59(2):263-72.
2. Clark-Kennedy AE. Quinidine in the treatment of auricular fibrillation. Quart J Med 1922;16:204-35.
3. Selzer A, Wray W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation 1964;30:17-26.
4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000;355:1048-52.
5. Hatta K, Takahashi T, Nakamura H, Yamashiro H, Yonezawa Y. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000;94(3):279-85.
6. Welch R. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60.
7. Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9.
8. Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J 1963;89:546-54.
9. Donatini B, LeBlaye I, Krupp P. Transient cardiac pacing is insufficiently used to treat arrhythmia associated with thioridazine. Cardiology 1992;81(6):340-1.
10. Fowler NO, McCall D, et al. Electrocardiographic changes and cardiac arrhythmias in patients receiving psychotropic drugs. Am J Cardiol 1976;37:223-30.
11. Mehtonen OP, Aranko K, Malkonen L, Vapaatalo H. A survey of sudden death associated with the use of antipsychotic or antidepressant drugs: 49 cases in Finland. Acta Psychiatr Scand 1991;84:58-64.
12 Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353-70.
13. Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol 1993;72(suppl):17B-22B.
14. Bednar MM, et al. The QT Interval. Prog Cardiovas Dis 2001;43(5, pt 2):1-45.
15. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12(4):411-20.
16. Browne K, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol 1983;52(1):55-9.
17. Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol 1991;67(8):774-6.
18. Woosley R, Sketch MH. Gender and drug-induced torsade de pointes. Bethesda, Md: American College of Cardiology, 1998; ACCEL 30, No. 2.
19. Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-6.
20. Vincent GM. Long QT syndrome. Cardiology Clinics 1999;18:309-25.
21. Khan SP, Dahlvani S, Vieweg WVR, Bernardo NL, Lewis RE. Electrocardiographic QT interval in a geropsychiatric inpatient population: a preliminary study. Med Psychiatr 1998;1:71-4.
22. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 1996;94(5):1018-22.
23. Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122(9):701-14.
24. Jackman WM, Friday KJ, Anderson JL, et al. The long QT syndromes: a critical review, new clinical observations, and a unifying hypothesis. Prog Cardiovas Dis 1988;31(2):115-72.
25. Hatta K, Takahashi T, Nakamura H, et al. Hypokalemia and agitation in acute psychotic patients. Psychiatry Res 1999;86(1):85-8.
26. Food and Drug Administration Advisory Committee: Zeldox capsules (ziprasidone): summary of efficacy and safety and overall benefit risk relationship. Bethesda, Md: Food and Drug Administration, July 19, 2000.