User login
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
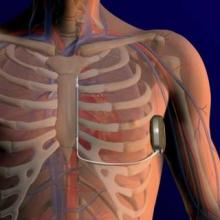
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: A subcutaneous ICD successfully converted ventricular arrhythmias in 100% of patients.
Data Source: A prospective, nonrandomized multicenter study was conducted.
Disclosures: The study was funded by Cameron Health, maker of the device. Dr. Burke and his coauthors have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.