User login
Watchman, Watch Out: Lariat Lassos Left Atrial Appendage
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
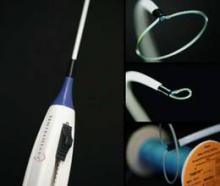
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
AT THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: In 82 of 89 patients (92%), the Lariat suture delivery device successfully closed the left atrial appendage down to a diameter of 1 mm or less.
Data Source: Data are from a single-center, nonrandomized study of 89 AF patients who were ineligible for warfarin.
Disclosures: The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
ICDs' Mortality Benefit Persists Up to 12 Years
BOSTON – More than a decade’s worth of follow-up of participants in the SCD-HeFT trial confirms that implantable cardioverter defibrillators in patients with moderate heart failure and reduced left ventricular systolic function can significantly reduce mortality, Dr. Jeanne Poole reported at the annual meeting of the Heart Rhythm Society.
ICD therapy was most beneficial in patients with New York Heart Association (NYHA) class II disease and ischemic heart failure, reported Dr. Poole, professor of medicine and director of the arrhythmia service and electrophysiology laboratory at the University of Washington in Seattle.
But as the original analysis of SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) showed, ICDs did not appear to benefit patients with NYHA class III disease, for whom cardiac resynchronization therapy (CRT) was not available at the time of enrollment (N. Engl. J. Med. 2005;352:225-37). Additionally, ICDs benefited patients with ischemic, but not nonischemic, heart failure, Dr. Poole noted.
Despite the significant reduction in mortality seen in some patients, "the mortality we observed at median follow-up of 11 years is substantial and reflects the reality of patients diagnosed at least a decade ago with heart failure," she said at a late-breaking abstracts session.
The SCD-HeFT trial was designed to see whether amiodarone (Cordarone, Pacerone) or a single-lead ICD, programmed conservatively to shock only, could reduce all-cause mortality compared with placebo in patients with ischemic or nonischemic NYHA class II-III heart failure with ejection fraction 35% or less.
In all, 829 patients were assigned to receive ICDs, 845 to amiodarone, and 847 to placebo during 1997-2001. The trial ended in October 2003.
An intention-to-treat analysis at 5 years (median follow-up 45.5 months) showed that although amiodarone was no better than placebo at preventing deaths, ICD treatment was associated with a 7.2% absolute risk reduction (hazard ratio, 0.77; P = .007).
The current analysis carried follow-up out an additional 5 or more years. The investigators contacted the 148 original trial enrollment sites asking for data on the patients. Two of the sites reported that all of the patients enrolled there had died, 110 others provided mortality data (89 included clinical or arrhythmia data), and 36 sites did not respond or chose not to participate.
Mortality data were available for 2,294 of the original 2,521 participants (91%).
The 12-year all-cause mortality for patients randomized to ICD treatment was 59%, compared with 64% for patients randomized to placebo (HR, 0.87; P = .028), translating into an absolute risk reduction of 5%.
Among patients with NYHA class II heart failure at enrollment, the all-cause mortality rate was significantly lower than among patients originally assigned to placebo (HR, 0.76; P = .001). However, patients with class III disease at enrollment did no better than did controls (HR, 1.06).
Similarly, patients with an ischemic heart failure etiology did better than did placebo patients (HR, 0.81; P = .001), but those with nonischemic origin did not.
Consistent with the observations in the original trial, amiodarone did not confer a survival benefit compared with placebo.
Study limitations include vital status determination on only 91% of the original participants, limited data on new ICD implants during follow-up, and limited data on long-term use of amiodarone. Additionally, "long-term mortality for patients in the original randomized treatment groups may have been confounded by multiple clinical and advanced heart failure therapies after SCD-HeFT was completed," Dr. Poole noted.
Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, said in an interview that the long-term data show that clinicians need better tools than just ejection fraction for determining which patients with heart failure are most at risk and could benefit from more aggressive interventions.
"SCD-HeFT showed a 5% absolute difference. It would be nice to find a group of indicators that would tell you who is really going to be at risk for arrhythmic death but live 10 years with their heart failure. Unfortunately, because they don’t have the updated information about therapy, it’s difficult to make a lot of interpretation of their results," she said.
Dr. Albert comoderated the session in which the data were presented, but was not involved in the study.
The study was funded by the National Heart, Lung, and Blood Institute with a subsidiary grant for St. Jude Medical Corp., maker of the ICD used in the study. Dr. Poole disclosed being on the speakers bureau for St. Jude Medical, Medtronic Inc., and Boston Scientific Corp. Dr. Albert disclosed receiving research support from St. Jude Medical.
BOSTON – More than a decade’s worth of follow-up of participants in the SCD-HeFT trial confirms that implantable cardioverter defibrillators in patients with moderate heart failure and reduced left ventricular systolic function can significantly reduce mortality, Dr. Jeanne Poole reported at the annual meeting of the Heart Rhythm Society.
ICD therapy was most beneficial in patients with New York Heart Association (NYHA) class II disease and ischemic heart failure, reported Dr. Poole, professor of medicine and director of the arrhythmia service and electrophysiology laboratory at the University of Washington in Seattle.
But as the original analysis of SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) showed, ICDs did not appear to benefit patients with NYHA class III disease, for whom cardiac resynchronization therapy (CRT) was not available at the time of enrollment (N. Engl. J. Med. 2005;352:225-37). Additionally, ICDs benefited patients with ischemic, but not nonischemic, heart failure, Dr. Poole noted.
Despite the significant reduction in mortality seen in some patients, "the mortality we observed at median follow-up of 11 years is substantial and reflects the reality of patients diagnosed at least a decade ago with heart failure," she said at a late-breaking abstracts session.
The SCD-HeFT trial was designed to see whether amiodarone (Cordarone, Pacerone) or a single-lead ICD, programmed conservatively to shock only, could reduce all-cause mortality compared with placebo in patients with ischemic or nonischemic NYHA class II-III heart failure with ejection fraction 35% or less.
In all, 829 patients were assigned to receive ICDs, 845 to amiodarone, and 847 to placebo during 1997-2001. The trial ended in October 2003.
An intention-to-treat analysis at 5 years (median follow-up 45.5 months) showed that although amiodarone was no better than placebo at preventing deaths, ICD treatment was associated with a 7.2% absolute risk reduction (hazard ratio, 0.77; P = .007).
The current analysis carried follow-up out an additional 5 or more years. The investigators contacted the 148 original trial enrollment sites asking for data on the patients. Two of the sites reported that all of the patients enrolled there had died, 110 others provided mortality data (89 included clinical or arrhythmia data), and 36 sites did not respond or chose not to participate.
Mortality data were available for 2,294 of the original 2,521 participants (91%).
The 12-year all-cause mortality for patients randomized to ICD treatment was 59%, compared with 64% for patients randomized to placebo (HR, 0.87; P = .028), translating into an absolute risk reduction of 5%.
Among patients with NYHA class II heart failure at enrollment, the all-cause mortality rate was significantly lower than among patients originally assigned to placebo (HR, 0.76; P = .001). However, patients with class III disease at enrollment did no better than did controls (HR, 1.06).
Similarly, patients with an ischemic heart failure etiology did better than did placebo patients (HR, 0.81; P = .001), but those with nonischemic origin did not.
Consistent with the observations in the original trial, amiodarone did not confer a survival benefit compared with placebo.
Study limitations include vital status determination on only 91% of the original participants, limited data on new ICD implants during follow-up, and limited data on long-term use of amiodarone. Additionally, "long-term mortality for patients in the original randomized treatment groups may have been confounded by multiple clinical and advanced heart failure therapies after SCD-HeFT was completed," Dr. Poole noted.
Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, said in an interview that the long-term data show that clinicians need better tools than just ejection fraction for determining which patients with heart failure are most at risk and could benefit from more aggressive interventions.
"SCD-HeFT showed a 5% absolute difference. It would be nice to find a group of indicators that would tell you who is really going to be at risk for arrhythmic death but live 10 years with their heart failure. Unfortunately, because they don’t have the updated information about therapy, it’s difficult to make a lot of interpretation of their results," she said.
Dr. Albert comoderated the session in which the data were presented, but was not involved in the study.
The study was funded by the National Heart, Lung, and Blood Institute with a subsidiary grant for St. Jude Medical Corp., maker of the ICD used in the study. Dr. Poole disclosed being on the speakers bureau for St. Jude Medical, Medtronic Inc., and Boston Scientific Corp. Dr. Albert disclosed receiving research support from St. Jude Medical.
BOSTON – More than a decade’s worth of follow-up of participants in the SCD-HeFT trial confirms that implantable cardioverter defibrillators in patients with moderate heart failure and reduced left ventricular systolic function can significantly reduce mortality, Dr. Jeanne Poole reported at the annual meeting of the Heart Rhythm Society.
ICD therapy was most beneficial in patients with New York Heart Association (NYHA) class II disease and ischemic heart failure, reported Dr. Poole, professor of medicine and director of the arrhythmia service and electrophysiology laboratory at the University of Washington in Seattle.
But as the original analysis of SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) showed, ICDs did not appear to benefit patients with NYHA class III disease, for whom cardiac resynchronization therapy (CRT) was not available at the time of enrollment (N. Engl. J. Med. 2005;352:225-37). Additionally, ICDs benefited patients with ischemic, but not nonischemic, heart failure, Dr. Poole noted.
Despite the significant reduction in mortality seen in some patients, "the mortality we observed at median follow-up of 11 years is substantial and reflects the reality of patients diagnosed at least a decade ago with heart failure," she said at a late-breaking abstracts session.
The SCD-HeFT trial was designed to see whether amiodarone (Cordarone, Pacerone) or a single-lead ICD, programmed conservatively to shock only, could reduce all-cause mortality compared with placebo in patients with ischemic or nonischemic NYHA class II-III heart failure with ejection fraction 35% or less.
In all, 829 patients were assigned to receive ICDs, 845 to amiodarone, and 847 to placebo during 1997-2001. The trial ended in October 2003.
An intention-to-treat analysis at 5 years (median follow-up 45.5 months) showed that although amiodarone was no better than placebo at preventing deaths, ICD treatment was associated with a 7.2% absolute risk reduction (hazard ratio, 0.77; P = .007).
The current analysis carried follow-up out an additional 5 or more years. The investigators contacted the 148 original trial enrollment sites asking for data on the patients. Two of the sites reported that all of the patients enrolled there had died, 110 others provided mortality data (89 included clinical or arrhythmia data), and 36 sites did not respond or chose not to participate.
Mortality data were available for 2,294 of the original 2,521 participants (91%).
The 12-year all-cause mortality for patients randomized to ICD treatment was 59%, compared with 64% for patients randomized to placebo (HR, 0.87; P = .028), translating into an absolute risk reduction of 5%.
Among patients with NYHA class II heart failure at enrollment, the all-cause mortality rate was significantly lower than among patients originally assigned to placebo (HR, 0.76; P = .001). However, patients with class III disease at enrollment did no better than did controls (HR, 1.06).
Similarly, patients with an ischemic heart failure etiology did better than did placebo patients (HR, 0.81; P = .001), but those with nonischemic origin did not.
Consistent with the observations in the original trial, amiodarone did not confer a survival benefit compared with placebo.
Study limitations include vital status determination on only 91% of the original participants, limited data on new ICD implants during follow-up, and limited data on long-term use of amiodarone. Additionally, "long-term mortality for patients in the original randomized treatment groups may have been confounded by multiple clinical and advanced heart failure therapies after SCD-HeFT was completed," Dr. Poole noted.
Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, said in an interview that the long-term data show that clinicians need better tools than just ejection fraction for determining which patients with heart failure are most at risk and could benefit from more aggressive interventions.
"SCD-HeFT showed a 5% absolute difference. It would be nice to find a group of indicators that would tell you who is really going to be at risk for arrhythmic death but live 10 years with their heart failure. Unfortunately, because they don’t have the updated information about therapy, it’s difficult to make a lot of interpretation of their results," she said.
Dr. Albert comoderated the session in which the data were presented, but was not involved in the study.
The study was funded by the National Heart, Lung, and Blood Institute with a subsidiary grant for St. Jude Medical Corp., maker of the ICD used in the study. Dr. Poole disclosed being on the speakers bureau for St. Jude Medical, Medtronic Inc., and Boston Scientific Corp. Dr. Albert disclosed receiving research support from St. Jude Medical.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: The 12-year all-cause mortality rate for patients randomized to ICD in the SCD-HeFT trial was 59%, compared with 64% for patients randomized to placebo (HR, 0.87; P = .028), translating into an absolute risk reduction of 5%.
Data Source: Study was a follow-up of mortality data on patients originally enrolled in a prospective randomized trial.
Disclosures: The study was funded by the NHLBI with a subsidiary grant for St. Jude Medical Corp, maker of the ICD used in the study. Dr. Poole disclosed being on the speakers bureaus for St. Jude Medical, Medtronic, and Boston Scientific. Dr. Albert disclosed receiving research support from St. Jude Medical.
Shift From Atrial Overdrive Pacing for AF Prevention Urged
BOSTON – Continuous atrial overdrive pacing does not prevent the development of new atrial fibrillation and is not a useful feature of pacemakers for patients with no history of AF, a researcher said at the annual meeting of the Heart Rhythm Society.
A secondary analysis of data from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) of atrial pacing in older patients with no history of AF showed that continuous atrial overdrive pacing (CAOP) had "no discernible effect" on the incidence of new atrial tachyarrhythmia, AF longer than 6 minutes, or AF burden, reported Dr. Stefan Hohnloser, director of clinical electrophysiology at J.W. Goethe University in Frankfurt, Germany.
Atrial preventive pacing "was associated with a high rate of crossover to alternate pacing mode, an increase in AF burden in patients with minimal ventricular pacing, more false-positive detection of atrial fibrillation by the pacemaker, and more frequent pacemaker generator replacement," Dr. Hohnloser said.
There have been 24 small or moderately sized studies of atrial preventive pacing in more than 10,000 patients, yet the results of those studies have been muddied by relatively short follow-up, the use of many different devices from different manufacturers, different pacing algorithms, and variations in atrial lead placements, hence the rationale for the secondary goal of ASSERT, he said.
ASSERT was a randomized study of 2,343 patients aged 65 and older with a history of hypertension but no history of AF or prior use of a vitamin K antagonist. The primary hypothesis that subclinical AF detected by pacemakers or implantable cardioverter defibrillators (ICDs) could predict increased risk of stroke or systemic embolism was borne out by the results.
The same could not be said, however, for the secondary hypothesis that CAOP could prevent development of AF and clinical end points, Dr. Hohnloser said.
After a 3-month run-in period to determine the presence or absence of subclinical AF, patients were randomized in a single-blind fashion to have the CAOP feature of their devices switched on or kept off. Patients were followed every 3 months. Independent adjudicators read all device-stored electrograms longer than 6 minutes.
Over 2.5 years of follow-up, there were no significant differences between the CAOP-on or -off groups in time to atrial tachyarrhythmia or to a composite clinical end point of stroke, myocardial infarction, cardiovascular death, systemic embolism, or heart-failure hospitalization, he said.
A subgroup analysis showed that atrial lead position, atrioventricular node disease with or without sinus node disease, sinus node disease alone, or history of heart failure were not significant predictors of treatment effect by randomization. However, patients who spent less than the median time in ventricular pacing at 6 months (59%) had significantly more of the primary outcome events, compared with patients who spent more than 59% of the time in ventricular pacing, he reported.
In all, 11.4% of patients assigned to CAOP on at study entry were crossed over to CAOP off, compared with only 1.0% of patients in the off group who were crossed over to continuous atrial overdrive pacing, a significant difference.
One or more false-positive AF detections occurred in 23% of patients with continuous pacing, compared with 7.7% of those with it turned off, for a significant relative risk of 2.99.
Pacemaker generator replacement was required in 4.4% of patients with CAOP on, compared with 2.5% of those with it off (relative risk, 1.70; P = .02). This result was expected, Dr. Hohnloser said, because of the extra workload on the pacemakers in continuous overdrive.
The results confirm that patients not already in atrial fibrillation do not appear to benefit from CAOP, but the study did not address whether the strategy benefits patients who already have AF, commented Dr. Richard I. Fogel from the St. Vincent Medical Group, Indianapolis, in an interview.
"The question didn’t address whether it decreases the atrial fibrillation burden. But I think it’s very clear that if you don’t have atrial fibrillation and you have had it, you shouldn’t use this algorithm. Although you have to wonder whether there aren’t some subsets of patients who might benefit," he said. Dr. Fogel moderated the late-breaking abstracts session but was not involved in the study.
The ASSERT trial was funded by St. Jude Medical. Dr. Hohnloser disclosed serving as a consultant, member of the steering committee, and speakers bureau member for St. Jude Medical and other companies. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant and owns stock in Medtronic and Guidant.
BOSTON – Continuous atrial overdrive pacing does not prevent the development of new atrial fibrillation and is not a useful feature of pacemakers for patients with no history of AF, a researcher said at the annual meeting of the Heart Rhythm Society.
A secondary analysis of data from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) of atrial pacing in older patients with no history of AF showed that continuous atrial overdrive pacing (CAOP) had "no discernible effect" on the incidence of new atrial tachyarrhythmia, AF longer than 6 minutes, or AF burden, reported Dr. Stefan Hohnloser, director of clinical electrophysiology at J.W. Goethe University in Frankfurt, Germany.
Atrial preventive pacing "was associated with a high rate of crossover to alternate pacing mode, an increase in AF burden in patients with minimal ventricular pacing, more false-positive detection of atrial fibrillation by the pacemaker, and more frequent pacemaker generator replacement," Dr. Hohnloser said.
There have been 24 small or moderately sized studies of atrial preventive pacing in more than 10,000 patients, yet the results of those studies have been muddied by relatively short follow-up, the use of many different devices from different manufacturers, different pacing algorithms, and variations in atrial lead placements, hence the rationale for the secondary goal of ASSERT, he said.
ASSERT was a randomized study of 2,343 patients aged 65 and older with a history of hypertension but no history of AF or prior use of a vitamin K antagonist. The primary hypothesis that subclinical AF detected by pacemakers or implantable cardioverter defibrillators (ICDs) could predict increased risk of stroke or systemic embolism was borne out by the results.
The same could not be said, however, for the secondary hypothesis that CAOP could prevent development of AF and clinical end points, Dr. Hohnloser said.
After a 3-month run-in period to determine the presence or absence of subclinical AF, patients were randomized in a single-blind fashion to have the CAOP feature of their devices switched on or kept off. Patients were followed every 3 months. Independent adjudicators read all device-stored electrograms longer than 6 minutes.
Over 2.5 years of follow-up, there were no significant differences between the CAOP-on or -off groups in time to atrial tachyarrhythmia or to a composite clinical end point of stroke, myocardial infarction, cardiovascular death, systemic embolism, or heart-failure hospitalization, he said.
A subgroup analysis showed that atrial lead position, atrioventricular node disease with or without sinus node disease, sinus node disease alone, or history of heart failure were not significant predictors of treatment effect by randomization. However, patients who spent less than the median time in ventricular pacing at 6 months (59%) had significantly more of the primary outcome events, compared with patients who spent more than 59% of the time in ventricular pacing, he reported.
In all, 11.4% of patients assigned to CAOP on at study entry were crossed over to CAOP off, compared with only 1.0% of patients in the off group who were crossed over to continuous atrial overdrive pacing, a significant difference.
One or more false-positive AF detections occurred in 23% of patients with continuous pacing, compared with 7.7% of those with it turned off, for a significant relative risk of 2.99.
Pacemaker generator replacement was required in 4.4% of patients with CAOP on, compared with 2.5% of those with it off (relative risk, 1.70; P = .02). This result was expected, Dr. Hohnloser said, because of the extra workload on the pacemakers in continuous overdrive.
The results confirm that patients not already in atrial fibrillation do not appear to benefit from CAOP, but the study did not address whether the strategy benefits patients who already have AF, commented Dr. Richard I. Fogel from the St. Vincent Medical Group, Indianapolis, in an interview.
"The question didn’t address whether it decreases the atrial fibrillation burden. But I think it’s very clear that if you don’t have atrial fibrillation and you have had it, you shouldn’t use this algorithm. Although you have to wonder whether there aren’t some subsets of patients who might benefit," he said. Dr. Fogel moderated the late-breaking abstracts session but was not involved in the study.
The ASSERT trial was funded by St. Jude Medical. Dr. Hohnloser disclosed serving as a consultant, member of the steering committee, and speakers bureau member for St. Jude Medical and other companies. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant and owns stock in Medtronic and Guidant.
BOSTON – Continuous atrial overdrive pacing does not prevent the development of new atrial fibrillation and is not a useful feature of pacemakers for patients with no history of AF, a researcher said at the annual meeting of the Heart Rhythm Society.
A secondary analysis of data from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) of atrial pacing in older patients with no history of AF showed that continuous atrial overdrive pacing (CAOP) had "no discernible effect" on the incidence of new atrial tachyarrhythmia, AF longer than 6 minutes, or AF burden, reported Dr. Stefan Hohnloser, director of clinical electrophysiology at J.W. Goethe University in Frankfurt, Germany.
Atrial preventive pacing "was associated with a high rate of crossover to alternate pacing mode, an increase in AF burden in patients with minimal ventricular pacing, more false-positive detection of atrial fibrillation by the pacemaker, and more frequent pacemaker generator replacement," Dr. Hohnloser said.
There have been 24 small or moderately sized studies of atrial preventive pacing in more than 10,000 patients, yet the results of those studies have been muddied by relatively short follow-up, the use of many different devices from different manufacturers, different pacing algorithms, and variations in atrial lead placements, hence the rationale for the secondary goal of ASSERT, he said.
ASSERT was a randomized study of 2,343 patients aged 65 and older with a history of hypertension but no history of AF or prior use of a vitamin K antagonist. The primary hypothesis that subclinical AF detected by pacemakers or implantable cardioverter defibrillators (ICDs) could predict increased risk of stroke or systemic embolism was borne out by the results.
The same could not be said, however, for the secondary hypothesis that CAOP could prevent development of AF and clinical end points, Dr. Hohnloser said.
After a 3-month run-in period to determine the presence or absence of subclinical AF, patients were randomized in a single-blind fashion to have the CAOP feature of their devices switched on or kept off. Patients were followed every 3 months. Independent adjudicators read all device-stored electrograms longer than 6 minutes.
Over 2.5 years of follow-up, there were no significant differences between the CAOP-on or -off groups in time to atrial tachyarrhythmia or to a composite clinical end point of stroke, myocardial infarction, cardiovascular death, systemic embolism, or heart-failure hospitalization, he said.
A subgroup analysis showed that atrial lead position, atrioventricular node disease with or without sinus node disease, sinus node disease alone, or history of heart failure were not significant predictors of treatment effect by randomization. However, patients who spent less than the median time in ventricular pacing at 6 months (59%) had significantly more of the primary outcome events, compared with patients who spent more than 59% of the time in ventricular pacing, he reported.
In all, 11.4% of patients assigned to CAOP on at study entry were crossed over to CAOP off, compared with only 1.0% of patients in the off group who were crossed over to continuous atrial overdrive pacing, a significant difference.
One or more false-positive AF detections occurred in 23% of patients with continuous pacing, compared with 7.7% of those with it turned off, for a significant relative risk of 2.99.
Pacemaker generator replacement was required in 4.4% of patients with CAOP on, compared with 2.5% of those with it off (relative risk, 1.70; P = .02). This result was expected, Dr. Hohnloser said, because of the extra workload on the pacemakers in continuous overdrive.
The results confirm that patients not already in atrial fibrillation do not appear to benefit from CAOP, but the study did not address whether the strategy benefits patients who already have AF, commented Dr. Richard I. Fogel from the St. Vincent Medical Group, Indianapolis, in an interview.
"The question didn’t address whether it decreases the atrial fibrillation burden. But I think it’s very clear that if you don’t have atrial fibrillation and you have had it, you shouldn’t use this algorithm. Although you have to wonder whether there aren’t some subsets of patients who might benefit," he said. Dr. Fogel moderated the late-breaking abstracts session but was not involved in the study.
The ASSERT trial was funded by St. Jude Medical. Dr. Hohnloser disclosed serving as a consultant, member of the steering committee, and speakers bureau member for St. Jude Medical and other companies. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant and owns stock in Medtronic and Guidant.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: Over 2.5 years of follow-up, there were no significant differences in time to atrial tachyarrhythmia or to a composite clinical end point of stroke, MI, cardiovascular death, systemic embolism, or heart failure hospitalization between patients with no history of atrial fibrillation who had pacemakers/ICDs set to continuous atrial overdrive pacing on or off.
Data Source: The substudy was a secondary analysis of data from the randomized, prospective ASSERT trial.
Disclosures: ASSERT was funded by St. Jude Medical. Dr. Hohnloser disclosed serving as a consultant, member of the steering committee, and speakers bureau member for St. Jude Medical and other companies. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant and owns stock in Medtronic and Guidant.
Longer ICD Detection Window Reduces Inappropriate Shocks
BOSTON – Tweaking implantable cardioverter defibrillator settings to lengthen the detection window is safe and significantly reduces inappropriate antitachycardia pacing and shocks, an investigator said at the annual meeting of the Heart Rhythm Society.
Patients with ICDs programmed with a number of intervals to detect (NID) of 30/40 beats had a 37% reduction in ventricular therapies (antitachycardia pacing and shocks), compared with patients with ICDs programmed with an NID of 18/24 beats, with no significant differences in syncope or deaths between the groups, reported Dr. Maurizio Gasparini of Istituto Clinico Humanitas IRCCS, Milan.
"This strategy is demonstrated to be safe and effective in reducing unnecessary ICD therapy, and increasing consequently the quality of life of these patients," Dr. Gasparini said on behalf of coinvestigators in the randomized ADVANCE III (Avoid Delivering Therapies for Nonsustained Arrhythmias in ICD Patients III) trial.
In a previous trial, Dr. Gasparini and colleagues showed that 66% of ventricular fibrillation (VF) episodes, and 91% of fast ventricular tachycardia (FVT) episodes terminated spontaneously within 30 beats (Eur. Heart. J. 2009;30:2758-67), yet two major ICD manufacturers still have nominal (in-the-box) settings of only 2-3 seconds for a VF detection window, potentially leading to unpleasant and unnecessary shocks, he said.
The ADVANCE III investigators enrolled 1,902 patients from 94 centers with single-chamber, dual-chamber, or cardiac resynchronization therapy-defibrillator (CRT-D) ICDs. In all, 891 of those assigned to NID 18/24 programming with antitachycardia pacing (ATP) during charging and 876 patients assigned to NID 30/40 with ATP had available clinical data for the primary end point: a 20% or greater reduction in ATP and shocks for spontaneous arrhythmia with a cycle length of 320 ms or less.
The patients were predominantly male (84% in each arm) with a mean age of 65. Nearly half of patients in each group had New York Heart Association class III or IV heart failure, and 60% had coronary artery disease. The mean left ventricular ejection fraction in each group was 30%.
The devices were implanted for primary prevention in about 75% of patients in each arm. About 40% had CRT-Ds, 31% had dual-chamber devices, and 29% had single-chamber ICDs.
At a median follow-up of 12 months in an intention-to-treat analysis, 97 patients assigned to NID 30/40 had experienced 346 therapies (ATP or shock deliveries), compared with 149 patients and 557 therapies in those assigned to NID 18/24 (incidence rate ratio [IRR] 0.63, P less than .001), meeting the primary end point.
A Kaplan-Meier analysis also showed that the longer detection window was significantly better at keeping patients therapy free over 12 months.
There were no significant differences in syncopal events, which occurred in 1.5% of patients in the 30/40 group, compared with 0.8% in the 18/24 group, or in deaths, which occurred in 5.1% of patients randomized to the 30/40 strategy, and 5.9% of those assigned to 18/24.
The results suggest that "in many cases the nominal ICD settings are probably too conservative," Dr. Gasparini said.
Session comoderator Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, challenged the safety findings, noting that the incidence of syncope in both treatment arms was extremely low.
Dr. Gasparini agreed, but noted that in each arm of the study population, about 20% of participants had experienced one or more syncopal episodes prior to device implantation.
"This was a population that theoretically may have a high incidence of syncope; nonetheless, we did not observe very high incidence of it," he said.
The study was supported by Medtronic. Dr. Gasparini reported having no conflicts of interest. Two of the study coauthors are Medtronic employees. Dr. Albert has previously received research support from St. Jude Medical and was a consultant to Novartis.
BOSTON – Tweaking implantable cardioverter defibrillator settings to lengthen the detection window is safe and significantly reduces inappropriate antitachycardia pacing and shocks, an investigator said at the annual meeting of the Heart Rhythm Society.
Patients with ICDs programmed with a number of intervals to detect (NID) of 30/40 beats had a 37% reduction in ventricular therapies (antitachycardia pacing and shocks), compared with patients with ICDs programmed with an NID of 18/24 beats, with no significant differences in syncope or deaths between the groups, reported Dr. Maurizio Gasparini of Istituto Clinico Humanitas IRCCS, Milan.
"This strategy is demonstrated to be safe and effective in reducing unnecessary ICD therapy, and increasing consequently the quality of life of these patients," Dr. Gasparini said on behalf of coinvestigators in the randomized ADVANCE III (Avoid Delivering Therapies for Nonsustained Arrhythmias in ICD Patients III) trial.
In a previous trial, Dr. Gasparini and colleagues showed that 66% of ventricular fibrillation (VF) episodes, and 91% of fast ventricular tachycardia (FVT) episodes terminated spontaneously within 30 beats (Eur. Heart. J. 2009;30:2758-67), yet two major ICD manufacturers still have nominal (in-the-box) settings of only 2-3 seconds for a VF detection window, potentially leading to unpleasant and unnecessary shocks, he said.
The ADVANCE III investigators enrolled 1,902 patients from 94 centers with single-chamber, dual-chamber, or cardiac resynchronization therapy-defibrillator (CRT-D) ICDs. In all, 891 of those assigned to NID 18/24 programming with antitachycardia pacing (ATP) during charging and 876 patients assigned to NID 30/40 with ATP had available clinical data for the primary end point: a 20% or greater reduction in ATP and shocks for spontaneous arrhythmia with a cycle length of 320 ms or less.
The patients were predominantly male (84% in each arm) with a mean age of 65. Nearly half of patients in each group had New York Heart Association class III or IV heart failure, and 60% had coronary artery disease. The mean left ventricular ejection fraction in each group was 30%.
The devices were implanted for primary prevention in about 75% of patients in each arm. About 40% had CRT-Ds, 31% had dual-chamber devices, and 29% had single-chamber ICDs.
At a median follow-up of 12 months in an intention-to-treat analysis, 97 patients assigned to NID 30/40 had experienced 346 therapies (ATP or shock deliveries), compared with 149 patients and 557 therapies in those assigned to NID 18/24 (incidence rate ratio [IRR] 0.63, P less than .001), meeting the primary end point.
A Kaplan-Meier analysis also showed that the longer detection window was significantly better at keeping patients therapy free over 12 months.
There were no significant differences in syncopal events, which occurred in 1.5% of patients in the 30/40 group, compared with 0.8% in the 18/24 group, or in deaths, which occurred in 5.1% of patients randomized to the 30/40 strategy, and 5.9% of those assigned to 18/24.
The results suggest that "in many cases the nominal ICD settings are probably too conservative," Dr. Gasparini said.
Session comoderator Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, challenged the safety findings, noting that the incidence of syncope in both treatment arms was extremely low.
Dr. Gasparini agreed, but noted that in each arm of the study population, about 20% of participants had experienced one or more syncopal episodes prior to device implantation.
"This was a population that theoretically may have a high incidence of syncope; nonetheless, we did not observe very high incidence of it," he said.
The study was supported by Medtronic. Dr. Gasparini reported having no conflicts of interest. Two of the study coauthors are Medtronic employees. Dr. Albert has previously received research support from St. Jude Medical and was a consultant to Novartis.
BOSTON – Tweaking implantable cardioverter defibrillator settings to lengthen the detection window is safe and significantly reduces inappropriate antitachycardia pacing and shocks, an investigator said at the annual meeting of the Heart Rhythm Society.
Patients with ICDs programmed with a number of intervals to detect (NID) of 30/40 beats had a 37% reduction in ventricular therapies (antitachycardia pacing and shocks), compared with patients with ICDs programmed with an NID of 18/24 beats, with no significant differences in syncope or deaths between the groups, reported Dr. Maurizio Gasparini of Istituto Clinico Humanitas IRCCS, Milan.
"This strategy is demonstrated to be safe and effective in reducing unnecessary ICD therapy, and increasing consequently the quality of life of these patients," Dr. Gasparini said on behalf of coinvestigators in the randomized ADVANCE III (Avoid Delivering Therapies for Nonsustained Arrhythmias in ICD Patients III) trial.
In a previous trial, Dr. Gasparini and colleagues showed that 66% of ventricular fibrillation (VF) episodes, and 91% of fast ventricular tachycardia (FVT) episodes terminated spontaneously within 30 beats (Eur. Heart. J. 2009;30:2758-67), yet two major ICD manufacturers still have nominal (in-the-box) settings of only 2-3 seconds for a VF detection window, potentially leading to unpleasant and unnecessary shocks, he said.
The ADVANCE III investigators enrolled 1,902 patients from 94 centers with single-chamber, dual-chamber, or cardiac resynchronization therapy-defibrillator (CRT-D) ICDs. In all, 891 of those assigned to NID 18/24 programming with antitachycardia pacing (ATP) during charging and 876 patients assigned to NID 30/40 with ATP had available clinical data for the primary end point: a 20% or greater reduction in ATP and shocks for spontaneous arrhythmia with a cycle length of 320 ms or less.
The patients were predominantly male (84% in each arm) with a mean age of 65. Nearly half of patients in each group had New York Heart Association class III or IV heart failure, and 60% had coronary artery disease. The mean left ventricular ejection fraction in each group was 30%.
The devices were implanted for primary prevention in about 75% of patients in each arm. About 40% had CRT-Ds, 31% had dual-chamber devices, and 29% had single-chamber ICDs.
At a median follow-up of 12 months in an intention-to-treat analysis, 97 patients assigned to NID 30/40 had experienced 346 therapies (ATP or shock deliveries), compared with 149 patients and 557 therapies in those assigned to NID 18/24 (incidence rate ratio [IRR] 0.63, P less than .001), meeting the primary end point.
A Kaplan-Meier analysis also showed that the longer detection window was significantly better at keeping patients therapy free over 12 months.
There were no significant differences in syncopal events, which occurred in 1.5% of patients in the 30/40 group, compared with 0.8% in the 18/24 group, or in deaths, which occurred in 5.1% of patients randomized to the 30/40 strategy, and 5.9% of those assigned to 18/24.
The results suggest that "in many cases the nominal ICD settings are probably too conservative," Dr. Gasparini said.
Session comoderator Dr. Christine M. Albert, director of the center for arrhythmia prevention at Brigham and Women’s Hospital in Boston, challenged the safety findings, noting that the incidence of syncope in both treatment arms was extremely low.
Dr. Gasparini agreed, but noted that in each arm of the study population, about 20% of participants had experienced one or more syncopal episodes prior to device implantation.
"This was a population that theoretically may have a high incidence of syncope; nonetheless, we did not observe very high incidence of it," he said.
The study was supported by Medtronic. Dr. Gasparini reported having no conflicts of interest. Two of the study coauthors are Medtronic employees. Dr. Albert has previously received research support from St. Jude Medical and was a consultant to Novartis.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: The incidence rate ratio of ICD therapy (antitachycardia pacing or shocks) was 37% lower among patients with implantable cardioverter defibrillators programmed to a longer arrhythmia detection interval (30/40 beats), compared with patients assigned to ICDs programmed to an 18/24-beat detection interval.
Data Source: This was a randomized prospective multicenter trial.
Disclosures: The study was supported by Medtronic. Dr. Gasparini reported having no conflicts of interest. Two of the study coauthors are Medtronic employees. Dr. Albert has previously received research support from St. Jude Medical and was a consultant to Novartis.
Guidance Offered on Children With Wolff-Parkinson-White Syndrome
BOSTON – Although it ranks behind hypertrophic cardiomyopathy as a cause of sudden cardiac death in children and young adults, the Wolff-Parkinson-White electrocardiogram pattern warrants monitoring and, in some cases, intervention, according to authors of a consensus statement announced at the annual meeting of the Heart Rhythm Society.
The Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) issued an expert consensus statement on the care of young, asymptomatic patients with the Wolff-Parkinson-White (WPW) electrocardiographic patterns, caused by an accessory cardiac electrical pathway.
The statement is intended as a guideline for clinicians who treat patients aged 8-21 years who have the WPW pattern but are otherwise asymptomatic, said lead author Dr. Mitchell I. Cohen, chief of pediatric cardiology and director of pediatric electrophysiology at Phoenix Children’s Hospital.
An estimated 65% of young patients with WPW are asymptomatic, Dr. Cohen said in a briefing. In those patients, "essentially one of three things can happen: They may remain asymptomatic; they may develop an arrhythmia that can be managed with medication or ablation; or, more concerning, they may have a life-threatening event and die suddenly. The incidence of sudden death is quite rare, but it’s not zero," he said.
The consensus panel, comprising both pediatric and adult electrophysiologists, estimates the prevalence of the WPW to range from 1 to 3 per 1,000. The incidence of sudden death from WPW, including resuscitated sudden cardiac death (SCD), is about 4.5 per 1,000 patient-years, on the basis of a study of asymptomatic adults with the pattern who were followed for a mean of 38 months (J. Am. Coll. Cardiol. 2003;41:239-44).
In contrast, the incidence of sudden death attributable to hypertrophic cardiomyopathy was about 7.4 per 1,000 person-years in one study. (N. Engl. J. Med. 2000;342:1778-85).
Symptoms of WPW may include palpitations, dizziness, syncope, and supraventricular tachycardia. Many young patients are diagnosed only after they undergo electrocardiograms required by many school districts prior to participation in organized sports.
Dr. Cohen says that although the condition can be effectively treated with catheter-based radiofrequency ablation, invasive techniques may not always be necessary or appropriate for younger patients.
Specifically, the statement recommends the following for patients aged 8-21 years who have the WPW ECG pattern:
• Patients should take an exercise stress test if the ambulatory ECG exhibits persistent pre-excitation.
• Invasive risk stratification (transesophageal or intracardiac) should be performed to assess the shortest pre-excited RR interval in atrial fibrillation in patients in whom noninvasive testing fails to demonstrate clear and abrupt loss of pre-excitation.
• Catheter ablation may be considered in young patients with a measurement of the SPERRI (Shortest Pre-Excited RR Interval) of 250 ms or less in atrial fibrillation, as they are at increased risk for SCD.
• Ablation may be safely deferred in lower-risk young patients with a SPERRI longer than 250 ms in atrial fibrillation.
• Catheter ablation may be considered in previously asymptomatic patients who subsequently develop cardiovascular symptoms such as syncope or palpitations.
• Ablation may be considered regardless of the anterograde characteristics of the accessory pathway in asymptomatic patients with a WPW ECG pattern and structural heart disease.
• Asymptomatic patients with a WPW ECG pattern and ventricular dysfunction secondary to dyssynchronous contractions, regardless of anterograde characteristics of the bypass tract, may benefit from ablation.
• It is safe to prescribe medications for attention-deficit/hyperactivity disorder (ADHD) for asymptomatic patients with a WPW ECG in accordance with American Heart Association guidelines, which state that ADHD medications may be used in this setting after cardiac evaluation and with intermittent monitoring and supervision by a pediatric cardiologist.
The consensus statement has been endorsed by the governing bodies of the PACES, the HRS, the American College of Cardiology Foundation, the American Heart Association, the American Academy of Pediatrics, and the Canadian Heart Rhythm Society.
Dr. Cohen reported having no relevant disclosures.
BOSTON – Although it ranks behind hypertrophic cardiomyopathy as a cause of sudden cardiac death in children and young adults, the Wolff-Parkinson-White electrocardiogram pattern warrants monitoring and, in some cases, intervention, according to authors of a consensus statement announced at the annual meeting of the Heart Rhythm Society.
The Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) issued an expert consensus statement on the care of young, asymptomatic patients with the Wolff-Parkinson-White (WPW) electrocardiographic patterns, caused by an accessory cardiac electrical pathway.
The statement is intended as a guideline for clinicians who treat patients aged 8-21 years who have the WPW pattern but are otherwise asymptomatic, said lead author Dr. Mitchell I. Cohen, chief of pediatric cardiology and director of pediatric electrophysiology at Phoenix Children’s Hospital.
An estimated 65% of young patients with WPW are asymptomatic, Dr. Cohen said in a briefing. In those patients, "essentially one of three things can happen: They may remain asymptomatic; they may develop an arrhythmia that can be managed with medication or ablation; or, more concerning, they may have a life-threatening event and die suddenly. The incidence of sudden death is quite rare, but it’s not zero," he said.
The consensus panel, comprising both pediatric and adult electrophysiologists, estimates the prevalence of the WPW to range from 1 to 3 per 1,000. The incidence of sudden death from WPW, including resuscitated sudden cardiac death (SCD), is about 4.5 per 1,000 patient-years, on the basis of a study of asymptomatic adults with the pattern who were followed for a mean of 38 months (J. Am. Coll. Cardiol. 2003;41:239-44).
In contrast, the incidence of sudden death attributable to hypertrophic cardiomyopathy was about 7.4 per 1,000 person-years in one study. (N. Engl. J. Med. 2000;342:1778-85).
Symptoms of WPW may include palpitations, dizziness, syncope, and supraventricular tachycardia. Many young patients are diagnosed only after they undergo electrocardiograms required by many school districts prior to participation in organized sports.
Dr. Cohen says that although the condition can be effectively treated with catheter-based radiofrequency ablation, invasive techniques may not always be necessary or appropriate for younger patients.
Specifically, the statement recommends the following for patients aged 8-21 years who have the WPW ECG pattern:
• Patients should take an exercise stress test if the ambulatory ECG exhibits persistent pre-excitation.
• Invasive risk stratification (transesophageal or intracardiac) should be performed to assess the shortest pre-excited RR interval in atrial fibrillation in patients in whom noninvasive testing fails to demonstrate clear and abrupt loss of pre-excitation.
• Catheter ablation may be considered in young patients with a measurement of the SPERRI (Shortest Pre-Excited RR Interval) of 250 ms or less in atrial fibrillation, as they are at increased risk for SCD.
• Ablation may be safely deferred in lower-risk young patients with a SPERRI longer than 250 ms in atrial fibrillation.
• Catheter ablation may be considered in previously asymptomatic patients who subsequently develop cardiovascular symptoms such as syncope or palpitations.
• Ablation may be considered regardless of the anterograde characteristics of the accessory pathway in asymptomatic patients with a WPW ECG pattern and structural heart disease.
• Asymptomatic patients with a WPW ECG pattern and ventricular dysfunction secondary to dyssynchronous contractions, regardless of anterograde characteristics of the bypass tract, may benefit from ablation.
• It is safe to prescribe medications for attention-deficit/hyperactivity disorder (ADHD) for asymptomatic patients with a WPW ECG in accordance with American Heart Association guidelines, which state that ADHD medications may be used in this setting after cardiac evaluation and with intermittent monitoring and supervision by a pediatric cardiologist.
The consensus statement has been endorsed by the governing bodies of the PACES, the HRS, the American College of Cardiology Foundation, the American Heart Association, the American Academy of Pediatrics, and the Canadian Heart Rhythm Society.
Dr. Cohen reported having no relevant disclosures.
BOSTON – Although it ranks behind hypertrophic cardiomyopathy as a cause of sudden cardiac death in children and young adults, the Wolff-Parkinson-White electrocardiogram pattern warrants monitoring and, in some cases, intervention, according to authors of a consensus statement announced at the annual meeting of the Heart Rhythm Society.
The Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) issued an expert consensus statement on the care of young, asymptomatic patients with the Wolff-Parkinson-White (WPW) electrocardiographic patterns, caused by an accessory cardiac electrical pathway.
The statement is intended as a guideline for clinicians who treat patients aged 8-21 years who have the WPW pattern but are otherwise asymptomatic, said lead author Dr. Mitchell I. Cohen, chief of pediatric cardiology and director of pediatric electrophysiology at Phoenix Children’s Hospital.
An estimated 65% of young patients with WPW are asymptomatic, Dr. Cohen said in a briefing. In those patients, "essentially one of three things can happen: They may remain asymptomatic; they may develop an arrhythmia that can be managed with medication or ablation; or, more concerning, they may have a life-threatening event and die suddenly. The incidence of sudden death is quite rare, but it’s not zero," he said.
The consensus panel, comprising both pediatric and adult electrophysiologists, estimates the prevalence of the WPW to range from 1 to 3 per 1,000. The incidence of sudden death from WPW, including resuscitated sudden cardiac death (SCD), is about 4.5 per 1,000 patient-years, on the basis of a study of asymptomatic adults with the pattern who were followed for a mean of 38 months (J. Am. Coll. Cardiol. 2003;41:239-44).
In contrast, the incidence of sudden death attributable to hypertrophic cardiomyopathy was about 7.4 per 1,000 person-years in one study. (N. Engl. J. Med. 2000;342:1778-85).
Symptoms of WPW may include palpitations, dizziness, syncope, and supraventricular tachycardia. Many young patients are diagnosed only after they undergo electrocardiograms required by many school districts prior to participation in organized sports.
Dr. Cohen says that although the condition can be effectively treated with catheter-based radiofrequency ablation, invasive techniques may not always be necessary or appropriate for younger patients.
Specifically, the statement recommends the following for patients aged 8-21 years who have the WPW ECG pattern:
• Patients should take an exercise stress test if the ambulatory ECG exhibits persistent pre-excitation.
• Invasive risk stratification (transesophageal or intracardiac) should be performed to assess the shortest pre-excited RR interval in atrial fibrillation in patients in whom noninvasive testing fails to demonstrate clear and abrupt loss of pre-excitation.
• Catheter ablation may be considered in young patients with a measurement of the SPERRI (Shortest Pre-Excited RR Interval) of 250 ms or less in atrial fibrillation, as they are at increased risk for SCD.
• Ablation may be safely deferred in lower-risk young patients with a SPERRI longer than 250 ms in atrial fibrillation.
• Catheter ablation may be considered in previously asymptomatic patients who subsequently develop cardiovascular symptoms such as syncope or palpitations.
• Ablation may be considered regardless of the anterograde characteristics of the accessory pathway in asymptomatic patients with a WPW ECG pattern and structural heart disease.
• Asymptomatic patients with a WPW ECG pattern and ventricular dysfunction secondary to dyssynchronous contractions, regardless of anterograde characteristics of the bypass tract, may benefit from ablation.
• It is safe to prescribe medications for attention-deficit/hyperactivity disorder (ADHD) for asymptomatic patients with a WPW ECG in accordance with American Heart Association guidelines, which state that ADHD medications may be used in this setting after cardiac evaluation and with intermittent monitoring and supervision by a pediatric cardiologist.
The consensus statement has been endorsed by the governing bodies of the PACES, the HRS, the American College of Cardiology Foundation, the American Heart Association, the American Academy of Pediatrics, and the Canadian Heart Rhythm Society.
Dr. Cohen reported having no relevant disclosures.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Ablation Safe, Effective for First-Line Atrial Fib Treatment
BOSTON – Ablation bested antiarrhythmic drugs at reducing the incidence of time to first recurrence of atrial arrhythmias in patients with paroxysmal atrial fibrillation, results of a randomized, multicenter trial showed.
In the study, radiofrequency catheter–based pulmonary vein isolation was associated with a 44% relative risk reduction, compared with antiarrhythmics drugs in the primary end point of time to first recurrence of symptomatic or asymptomatic atrial fibrillation (AF), atrial tachyarrhythmia (AT), or atrial flutter (AFL), reported Dr. Carlos A. Morillo, coprincipal investigator of the RAAFT-2 (Radiofrequency Ablation vs. Antiarrhythmic Drugs as First-Line Treatment of Symptomatic Atrial Fibrillation) study at the annual meeting of the Heart Rhythm Society.
"Radiofrequency catheter pulmonary vein isolation achieved a significant reduction in all primary efficacy outcomes and most secondary outcomes with similar rates of success," said Dr. Morillo, a professor of cardiology at McMaster University in Hamilton, Ont.
"It certainly is very impressive that in these patients who for the first time have atrial fibrillation, they can do better with a strategy of ablation as a first-line therapy, compared to antiarrhythmic drugs. It’s only one trial, however, and it has to be validated, and we have to see how it carries forward, not only at the 1- or 2-year mark but over the long term," Dr. Richard I. Fogel of the St. Vincent Medical Group, Indianapolis, commented in an interview.
Dr. Fogel moderated the late-breaking abstract session at which these data were presented but was not involved in the study.
Previous studies have shown that ablation of atrial fibrillation results in about a 66% relative risk reduction in recurrence, but most studies have focused on patients with atrial fibrillation refractory to one or more antiarrhythmic drugs, Dr. Morillo noted.
The RAAFT-2 investigators enrolled 127 patients with symptomatic, recurrent paroxysmal AF lasting more than 30 seconds who had at least four episodes within the prior 6 months, with at least one of the episodes documented by Holter monitor, 12-lead ECG, event monitor, or rhythm strip.
In the intention-to-treat population, 66 patients (mean age, 56.3 years) were assigned to receive ablation, and 61 (mean age, 54.3 years) were allocated to receive antiarrhythmic drugs for treatment and follow-up, including flecainide (Tambocor), propafenone (Rythmol), dronedarone (Multaq), amiodarone (Cordarone), dofetilide (Tikosyn), and sotalol (Betapace).
About three-fourths of patients in each group were men, and about 87% had paroxysmal AF, with the remaining patients having persistent AF.
Among patients assigned to ablation, the mean number of AF episodes before enrollment was 47.7, and among patients assigned to antiarrhythmic drugs, enrollment was 33.
In the ablation group, 65 of 66 had the ablation performed. During follow-up one patient was lost to follow-up, nine received a second ablation, and seven were crossed over to antiarrhythmic drugs. In the drug group, 60 of 61 were started on drugs. One patient in this group was also lost follow-up, 36 discontinued antiarrhythmic drugs, and 26 were crossed over to ablation.
At 2-year follow-up, 72% of patients in the antiarrhythmic drug group reached the primary efficacy outcome (time to first recurrence of symptomatic or asymptomatic AF/AT/AFL), compared with 55% in the catheter ablation group, for a statistically significant risk reduction of 44%.
Looking at symptomatic AF/AT/AFL only, the proportion of patients with a first recurrence at 2 years was 59% and 47% for the ablation and medical therapy groups, respectively, for a significant 48% relative risk reduction.
In an analysis conducted to determine whether the interventions reduced the frequency of the primary outcome, the authors compared the percentage of transtelephonic monitor (TTM) transmissions indicating any recurrence of the arrhythmias. In all, 6.6% of transmissions in catheter ablation patients showed recurrence, compared with 14.7% of transmission from the antiarrhythmic drug group, yielding a highly significant risk reduction of 66% (P = .0001).
"Of note, when we excluded the transtelephonic monitor, we couldn’t show any [significant] difference in recurrence of the primary outcome – 31% in the antiarrhythmic drug and 24% in the catheter ablation – highlighting the need of very strict monitoring in these patients to be able to define a successful outcome."
Reported patient quality of life in both groups improved over baseline, and was not significantly different between the groups.
For the primary safety end point, there were no deaths in either group at 2 years. Cardiac tamponade was seen in 6.2% of patients in the ablation group, and severe pulmonary stenosis of 70% or greater in 1.5%. There were no cases of atrioesophageal fistula, thromboembolism, vascular complications, or phrenic nerve injury.
For patients on antiarrhythmic drugs, syncope occurred in 3.3%, atrial flutter with 1:1 conduction in 1.6%, and other significant advents leading to discontinuation of drug therapy in 14.3%.
The trial was sponsored by the Population Health Research Institute and McMaster University and Hamilton Health Sciences. It was supported by grant-in-aid from Biosense Webster. Dr. Morillo disclosed receiving consulting fees/honoraria/research grants from and/or being on a speakers bureau for Boehringer Ingelheim, Sanofi, Medtronic, Merck, St. Jude Medical, Boston Scientific, and Biosense Webster. Dr. Fogel disclosed that he has received grants for clinical research and educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – Ablation bested antiarrhythmic drugs at reducing the incidence of time to first recurrence of atrial arrhythmias in patients with paroxysmal atrial fibrillation, results of a randomized, multicenter trial showed.
In the study, radiofrequency catheter–based pulmonary vein isolation was associated with a 44% relative risk reduction, compared with antiarrhythmics drugs in the primary end point of time to first recurrence of symptomatic or asymptomatic atrial fibrillation (AF), atrial tachyarrhythmia (AT), or atrial flutter (AFL), reported Dr. Carlos A. Morillo, coprincipal investigator of the RAAFT-2 (Radiofrequency Ablation vs. Antiarrhythmic Drugs as First-Line Treatment of Symptomatic Atrial Fibrillation) study at the annual meeting of the Heart Rhythm Society.
"Radiofrequency catheter pulmonary vein isolation achieved a significant reduction in all primary efficacy outcomes and most secondary outcomes with similar rates of success," said Dr. Morillo, a professor of cardiology at McMaster University in Hamilton, Ont.
"It certainly is very impressive that in these patients who for the first time have atrial fibrillation, they can do better with a strategy of ablation as a first-line therapy, compared to antiarrhythmic drugs. It’s only one trial, however, and it has to be validated, and we have to see how it carries forward, not only at the 1- or 2-year mark but over the long term," Dr. Richard I. Fogel of the St. Vincent Medical Group, Indianapolis, commented in an interview.
Dr. Fogel moderated the late-breaking abstract session at which these data were presented but was not involved in the study.
Previous studies have shown that ablation of atrial fibrillation results in about a 66% relative risk reduction in recurrence, but most studies have focused on patients with atrial fibrillation refractory to one or more antiarrhythmic drugs, Dr. Morillo noted.
The RAAFT-2 investigators enrolled 127 patients with symptomatic, recurrent paroxysmal AF lasting more than 30 seconds who had at least four episodes within the prior 6 months, with at least one of the episodes documented by Holter monitor, 12-lead ECG, event monitor, or rhythm strip.
In the intention-to-treat population, 66 patients (mean age, 56.3 years) were assigned to receive ablation, and 61 (mean age, 54.3 years) were allocated to receive antiarrhythmic drugs for treatment and follow-up, including flecainide (Tambocor), propafenone (Rythmol), dronedarone (Multaq), amiodarone (Cordarone), dofetilide (Tikosyn), and sotalol (Betapace).
About three-fourths of patients in each group were men, and about 87% had paroxysmal AF, with the remaining patients having persistent AF.
Among patients assigned to ablation, the mean number of AF episodes before enrollment was 47.7, and among patients assigned to antiarrhythmic drugs, enrollment was 33.
In the ablation group, 65 of 66 had the ablation performed. During follow-up one patient was lost to follow-up, nine received a second ablation, and seven were crossed over to antiarrhythmic drugs. In the drug group, 60 of 61 were started on drugs. One patient in this group was also lost follow-up, 36 discontinued antiarrhythmic drugs, and 26 were crossed over to ablation.
At 2-year follow-up, 72% of patients in the antiarrhythmic drug group reached the primary efficacy outcome (time to first recurrence of symptomatic or asymptomatic AF/AT/AFL), compared with 55% in the catheter ablation group, for a statistically significant risk reduction of 44%.
Looking at symptomatic AF/AT/AFL only, the proportion of patients with a first recurrence at 2 years was 59% and 47% for the ablation and medical therapy groups, respectively, for a significant 48% relative risk reduction.
In an analysis conducted to determine whether the interventions reduced the frequency of the primary outcome, the authors compared the percentage of transtelephonic monitor (TTM) transmissions indicating any recurrence of the arrhythmias. In all, 6.6% of transmissions in catheter ablation patients showed recurrence, compared with 14.7% of transmission from the antiarrhythmic drug group, yielding a highly significant risk reduction of 66% (P = .0001).
"Of note, when we excluded the transtelephonic monitor, we couldn’t show any [significant] difference in recurrence of the primary outcome – 31% in the antiarrhythmic drug and 24% in the catheter ablation – highlighting the need of very strict monitoring in these patients to be able to define a successful outcome."
Reported patient quality of life in both groups improved over baseline, and was not significantly different between the groups.
For the primary safety end point, there were no deaths in either group at 2 years. Cardiac tamponade was seen in 6.2% of patients in the ablation group, and severe pulmonary stenosis of 70% or greater in 1.5%. There were no cases of atrioesophageal fistula, thromboembolism, vascular complications, or phrenic nerve injury.
For patients on antiarrhythmic drugs, syncope occurred in 3.3%, atrial flutter with 1:1 conduction in 1.6%, and other significant advents leading to discontinuation of drug therapy in 14.3%.
The trial was sponsored by the Population Health Research Institute and McMaster University and Hamilton Health Sciences. It was supported by grant-in-aid from Biosense Webster. Dr. Morillo disclosed receiving consulting fees/honoraria/research grants from and/or being on a speakers bureau for Boehringer Ingelheim, Sanofi, Medtronic, Merck, St. Jude Medical, Boston Scientific, and Biosense Webster. Dr. Fogel disclosed that he has received grants for clinical research and educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – Ablation bested antiarrhythmic drugs at reducing the incidence of time to first recurrence of atrial arrhythmias in patients with paroxysmal atrial fibrillation, results of a randomized, multicenter trial showed.
In the study, radiofrequency catheter–based pulmonary vein isolation was associated with a 44% relative risk reduction, compared with antiarrhythmics drugs in the primary end point of time to first recurrence of symptomatic or asymptomatic atrial fibrillation (AF), atrial tachyarrhythmia (AT), or atrial flutter (AFL), reported Dr. Carlos A. Morillo, coprincipal investigator of the RAAFT-2 (Radiofrequency Ablation vs. Antiarrhythmic Drugs as First-Line Treatment of Symptomatic Atrial Fibrillation) study at the annual meeting of the Heart Rhythm Society.
"Radiofrequency catheter pulmonary vein isolation achieved a significant reduction in all primary efficacy outcomes and most secondary outcomes with similar rates of success," said Dr. Morillo, a professor of cardiology at McMaster University in Hamilton, Ont.
"It certainly is very impressive that in these patients who for the first time have atrial fibrillation, they can do better with a strategy of ablation as a first-line therapy, compared to antiarrhythmic drugs. It’s only one trial, however, and it has to be validated, and we have to see how it carries forward, not only at the 1- or 2-year mark but over the long term," Dr. Richard I. Fogel of the St. Vincent Medical Group, Indianapolis, commented in an interview.
Dr. Fogel moderated the late-breaking abstract session at which these data were presented but was not involved in the study.
Previous studies have shown that ablation of atrial fibrillation results in about a 66% relative risk reduction in recurrence, but most studies have focused on patients with atrial fibrillation refractory to one or more antiarrhythmic drugs, Dr. Morillo noted.
The RAAFT-2 investigators enrolled 127 patients with symptomatic, recurrent paroxysmal AF lasting more than 30 seconds who had at least four episodes within the prior 6 months, with at least one of the episodes documented by Holter monitor, 12-lead ECG, event monitor, or rhythm strip.
In the intention-to-treat population, 66 patients (mean age, 56.3 years) were assigned to receive ablation, and 61 (mean age, 54.3 years) were allocated to receive antiarrhythmic drugs for treatment and follow-up, including flecainide (Tambocor), propafenone (Rythmol), dronedarone (Multaq), amiodarone (Cordarone), dofetilide (Tikosyn), and sotalol (Betapace).
About three-fourths of patients in each group were men, and about 87% had paroxysmal AF, with the remaining patients having persistent AF.
Among patients assigned to ablation, the mean number of AF episodes before enrollment was 47.7, and among patients assigned to antiarrhythmic drugs, enrollment was 33.
In the ablation group, 65 of 66 had the ablation performed. During follow-up one patient was lost to follow-up, nine received a second ablation, and seven were crossed over to antiarrhythmic drugs. In the drug group, 60 of 61 were started on drugs. One patient in this group was also lost follow-up, 36 discontinued antiarrhythmic drugs, and 26 were crossed over to ablation.
At 2-year follow-up, 72% of patients in the antiarrhythmic drug group reached the primary efficacy outcome (time to first recurrence of symptomatic or asymptomatic AF/AT/AFL), compared with 55% in the catheter ablation group, for a statistically significant risk reduction of 44%.
Looking at symptomatic AF/AT/AFL only, the proportion of patients with a first recurrence at 2 years was 59% and 47% for the ablation and medical therapy groups, respectively, for a significant 48% relative risk reduction.
In an analysis conducted to determine whether the interventions reduced the frequency of the primary outcome, the authors compared the percentage of transtelephonic monitor (TTM) transmissions indicating any recurrence of the arrhythmias. In all, 6.6% of transmissions in catheter ablation patients showed recurrence, compared with 14.7% of transmission from the antiarrhythmic drug group, yielding a highly significant risk reduction of 66% (P = .0001).
"Of note, when we excluded the transtelephonic monitor, we couldn’t show any [significant] difference in recurrence of the primary outcome – 31% in the antiarrhythmic drug and 24% in the catheter ablation – highlighting the need of very strict monitoring in these patients to be able to define a successful outcome."
Reported patient quality of life in both groups improved over baseline, and was not significantly different between the groups.
For the primary safety end point, there were no deaths in either group at 2 years. Cardiac tamponade was seen in 6.2% of patients in the ablation group, and severe pulmonary stenosis of 70% or greater in 1.5%. There were no cases of atrioesophageal fistula, thromboembolism, vascular complications, or phrenic nerve injury.
For patients on antiarrhythmic drugs, syncope occurred in 3.3%, atrial flutter with 1:1 conduction in 1.6%, and other significant advents leading to discontinuation of drug therapy in 14.3%.
The trial was sponsored by the Population Health Research Institute and McMaster University and Hamilton Health Sciences. It was supported by grant-in-aid from Biosense Webster. Dr. Morillo disclosed receiving consulting fees/honoraria/research grants from and/or being on a speakers bureau for Boehringer Ingelheim, Sanofi, Medtronic, Merck, St. Jude Medical, Boston Scientific, and Biosense Webster. Dr. Fogel disclosed that he has received grants for clinical research and educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: Radiofrequency catheter–based pulmonary vein isolation was associated with a 44% relative risk reduction, compared with antiarrhythmics drugs, in the primary end point of time to first recurrence of symptomatic or asymptomatic atrial fibrillation, atrial tachyarrhythmia, or atrial flutter over 2 years of follow-up.
Data Source: Data were taken from the RAAFT-2 randomized multicenter trial in 127 patients with symptomatic, recurrent paroxysmal atrial fibrillation.
Disclosures: The trial was sponsored by the Population Health Research Institute and McMaster University and Hamilton Health Sciences. It was supported by grant-in-aid from Biosense Webster. Dr. Morillo disclosed receiving consulting fees/honoraria/research grants from and/or being on a speakers bureau for Boehringer Ingelheim, Sanofi, Medtronic, Merck, St. Jude Medical, Boston Scientific, and Biosense Webster. Dr. Fogel disclosed that he has received grants for clinical research and educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
Subcutaneous ICD Passes Efficacy, Safety Muster
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
BOSTON – A subcutaneous implantable cardioverter defibrillator that does not require a transvenous lead appears to be safe and effective for the treatment of ventricular tachyarrhythmias, according to a prospective, nonrandomized multicenter study.
In the trial, the device (S-ICD system, Cameron Health) met its primary effectiveness end point of successful ventricular fibrillation – defined as two consecutive successful conversions out of a possible four attempts in the same shock polarity – in all of 304 evaluable patients, Dr. Martin C. Burke, director of the heart rhythm center at the University of Chicago, reported at the annual meeting of the Heart Rhythm Society.
The rates of major complications related to device implantation were 4.4% at 30 days and 7.9% at 180 days.
"This is not a niche device. We actually implanted it in anybody who had an indication for ICD implantation that met the guidelines indications overall," he said.
Unlike ICDs with transvenous leads, however, the subcutaneous device cannot provide pacing, Dr. Burke noted.
Dr. Hugh Calkins, who was not involved in the study, said in an interview that "the data look really terrific. What’s striking is that when this company started, [ICD] lead failures and lead problems weren’t paid much attention, and now this device is coming along just at a time when everyone is being reminded the implanted lead is the weak link of an implantable device of any type."
The device, if approved in the United States, would most benefit younger patients who do not need antitachycardial pacing, such as those with long QT or Brugada syndromes, or with hypertrophic cardiomyopathy, said Dr. Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute in Baltimore and vice president of the Heart Rhythm Society.
The device involves a pulse generator implanted into the left lateral, middle axillary line with a lead tunneled across to a subxiphoid incision, then carried along the left parasternum to the sternal angle (angle of Louis) at the second intercostal space. The lead has two electrodes: one that sits at the angle, and the other down by the xiphoid process, with a coil between the electrodes where the shock vector is continued across to the casing of the generator, or vice versa.
The investigators enrolled 330 patients with any standard indication for an ICD who did not require pacing. The patients came from 33 sites in the United States, New Zealand, the Netherlands, and the United Kingdom.
Common comorbidities in the group included heart failure in 61%, hypertension in 58%, myocardial infarction in 41%, and diabetes in 28%. Nearly one-third of patients (29%) had undergone percutaneous revascularization, 15% had a coronary artery bypass graft, and 13% had previously had a transvenous ICD.
Among the 321 patients in whom the implantation was attempted, the device was indicated for primary prevention in 79% and secondary prevention in 21%, similar to the proportion in the National Cardiovascular Data Registry, Dr. Burke said.
In all but 5% of the implantations, the device was inserted using only anatomical landmarks, with no medical imaging required.
Of the 321 patients, acute induction testing was not performed in 1, and was not evaluable in 16 patients, because they did not complete four required shock episodes, leaving 304 patients for the effectiveness analysis.
The device successfully converted in 100% of the 304 patients. In a sensitivity analysis including 11 additional patients with incomplete testing and one or more failed shocks, the conversion rate was 96.5%.
In a "worst-case" sensitivity analysis, including all nonevaluable patients, the successful conversion rate was 94.7%, above the prespecified lower boundary of a two-sided 95% confidence interval of more than 88%, Dr. Burke said.
There were 109 spontaneous episodes in 16 patients, with 1 patient experiencing a ventricular fibrillation storm of 81 episodes. All the episodes converted either spontaneously after the first shock, or with 80 J of energy.
The device algorithm prevents delivery of therapy for ventricular tachycardia/ventricular fibrillation rhythms that are likely to spontaneously terminate. Therapy was avoided in 63% if patients with VT/VF met criteria to charge the device without any reports of syncope, Dr. Burke said.
The safety analysis showed that the rate of freedom from type I complications (device-related complications requiring invasive interaction) was 99% at 180 days, above the performance goal of 79%. The rate of freedom from all device-, labeling-, and procedure-related complications at 180 days was 92%.
There were 18 suspected or confirmed infections, 14 of which were superficial or incisional infections successfully managed with antibiotics in 13 and sternal wound revision in 1, and 4 cases in which explantation of the device was required.
Inappropriate shocks occurred in 38 patients, 15 with supraventricular tachycardia in the shock-only zone, and 24 patients from oversensing (1 patient had multiple events). No patient had a shock caused by a discrimination error in the conditional shock zone.
Inappropriate shocks were reduced with dual-zone programming, Dr. Burke noted.
The device is currently approved for marketing in Europe (CE Marking) but has not received the Food and Drug Administration’s approval.
The study was funded by Cameron Health, maker of the device. Dr. Burke and his colleagues have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: A subcutaneous ICD successfully converted ventricular arrhythmias in 100% of patients.
Data Source: A prospective, nonrandomized multicenter study was conducted.
Disclosures: The study was funded by Cameron Health, maker of the device. Dr. Burke and his coauthors have received consulting fees, honoraria, and/or research grants from the company. Dr. Calkins reported having no relevant financial disclosures.
Independent Study Confirms Higher Riata ICD Lead Failure Rate
BOSTON – An independent investigation by seven U.S. centers has shown that recently recalled Riata implantable cardioverter defibrillator leads are significantly more prone to failure than are leads made by a different manufacturer.
A retrospective study of all patients older than 18 years who received ICDs with either Riata or Riata ST leads (St. Jude Medical) showed a failure rate of 1.93% per patient-year for Riata leads, significantly more than the rate of 0.43% for the comparator lead, Quattro Secure (Medtronic) (P less than .0001). The failure rate for Riata ST leads was 0.50%, a nonsignificant difference, reported Dr. Raed Abdelhadi of the Minneapolis Heart Institute.
The Riata and Riata ST ICD leads are prone to inside-out and outside-in silicone insulation defects, which can cause lead malfunction and externalization (breach of the outer insulation, visible outside the lead body) of the conductor cables, but until this study, there were no independent, multicenter investigations into the rate of lead failure, failure signs, or clinical sequelae, he said.
"Without such data, it is not possible to design evidence-based management strategies or to advise Riata patients of their risks," he said at the annual meeting of the Heart Rhythm Society.
Investigators at the seven centers looked at all-cause failures and electrical malfunctions in 774 patients who received an 8-French Riata lead (mean follow-up, 4.2 years), and 307 patients who received a 7-French Riata ST lead (mean follow-up, 3.3 years). ICD leads with silicone-polyurethane copolymer insulation (RIATA ST Optim and Durata leads) were excluded.
For comparison, they obtained data on the Quattro Secure ICD leads from a three-center study of the longevity and risk factors for failure of Sprint Fidelis leads (Circulation 2011;123:358-63).
The investigators defined lead failure as either "abnormal impedance leading to replacement, electrical noise manifested by nonphysiologic signals, increase in pacing threshold or decline in R-wave amplitude necessitating lead placement, inability to provide effective therapy due to apparent lead abnormality, or externalized conductor, clearly demonstrated on fluoroscopy or x-ray ... even if the lead was functioning normally and electrically intact."
Neither functional abnormalities (for example, exit block and physiologic oversensing in the presence of an electrically intact lead) nor lead displacement (unless caused by a bad fixation mechanism) were considered to be failures.
Of the 1,081 Riata and Riata ST leads implanted, 712 (66%) were active and functioning normally at last follow-up. Of the 302 (28%) removals from service, 266 were owing to death, 10 to transplant, 5 to perforation, 4 to infection, and 17 to other causes.
In all, 10% of leads in the study were examined for externalized conductors, and 27 (25%) were found to have them. Of this group, seven (26%) leads were malfunctioning.
There were 65 Riata failures and 5 Riata ST failures, for a 6.2% incidence of all-cause failures. Of this group, 47 failures were due to electrical malfunctions, including seven from the externalized conductors contributing to the malfunction, as noted before. An additional 20 leads had externalized conductors with normal function, but met the study definition of failure.
In contrast, there were 23 lead failures among 1,668 patients with Quattro Secure leads (1.37%).
In univariate analysis, neither age, gender, primary or secondary indication for ICD implantation, ejection fraction, type of cardiac disease, nor lead placement location were significantly associated with risk for lead failure. The same was true when the Riata ST leads were subtracted from the equation.
Signs of failure included noise resulting in inappropriate therapy, elevated thresholds requiring lead replacement resulting in loss of capture and syncope, abnormal pacing impedance, abnormal high-voltage impedance, decline in R value requiring lead replacement, and failed defibrillation threshold testing with abnormal high-voltage impedance, requiring lead replacement.
Dr. Abdelhadi noted that the study was limited by its retrospective design, the fact that only 10% of leads were examined for externalized conductors, and the relatively short follow-up in the Riata ST group compared with the other two groups. In addition, most lead failures were not confirmed by returned-product analysis.
The study confirms what was already known and what many meeting attendees expected to hear, commented Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, in an interview.
"The bottom line is that it’s all about the patients, and how we take care of them, advise them, and whether we explant the leads. The whole international heart rhythm community is focused on this question and what to do so that there aren’t deaths that result from noise, that there aren’t deaths from unnecessary lead extractions," he said. Dr. Calkins was not involved in the study, but he moderated a media briefing where Dr. Abdelhadi presented the data.
Dr. Abdelhadi and Dr. Calkins reported having no current conflicts of interest to disclose.
BOSTON – An independent investigation by seven U.S. centers has shown that recently recalled Riata implantable cardioverter defibrillator leads are significantly more prone to failure than are leads made by a different manufacturer.
A retrospective study of all patients older than 18 years who received ICDs with either Riata or Riata ST leads (St. Jude Medical) showed a failure rate of 1.93% per patient-year for Riata leads, significantly more than the rate of 0.43% for the comparator lead, Quattro Secure (Medtronic) (P less than .0001). The failure rate for Riata ST leads was 0.50%, a nonsignificant difference, reported Dr. Raed Abdelhadi of the Minneapolis Heart Institute.
The Riata and Riata ST ICD leads are prone to inside-out and outside-in silicone insulation defects, which can cause lead malfunction and externalization (breach of the outer insulation, visible outside the lead body) of the conductor cables, but until this study, there were no independent, multicenter investigations into the rate of lead failure, failure signs, or clinical sequelae, he said.
"Without such data, it is not possible to design evidence-based management strategies or to advise Riata patients of their risks," he said at the annual meeting of the Heart Rhythm Society.
Investigators at the seven centers looked at all-cause failures and electrical malfunctions in 774 patients who received an 8-French Riata lead (mean follow-up, 4.2 years), and 307 patients who received a 7-French Riata ST lead (mean follow-up, 3.3 years). ICD leads with silicone-polyurethane copolymer insulation (RIATA ST Optim and Durata leads) were excluded.
For comparison, they obtained data on the Quattro Secure ICD leads from a three-center study of the longevity and risk factors for failure of Sprint Fidelis leads (Circulation 2011;123:358-63).
The investigators defined lead failure as either "abnormal impedance leading to replacement, electrical noise manifested by nonphysiologic signals, increase in pacing threshold or decline in R-wave amplitude necessitating lead placement, inability to provide effective therapy due to apparent lead abnormality, or externalized conductor, clearly demonstrated on fluoroscopy or x-ray ... even if the lead was functioning normally and electrically intact."
Neither functional abnormalities (for example, exit block and physiologic oversensing in the presence of an electrically intact lead) nor lead displacement (unless caused by a bad fixation mechanism) were considered to be failures.
Of the 1,081 Riata and Riata ST leads implanted, 712 (66%) were active and functioning normally at last follow-up. Of the 302 (28%) removals from service, 266 were owing to death, 10 to transplant, 5 to perforation, 4 to infection, and 17 to other causes.
In all, 10% of leads in the study were examined for externalized conductors, and 27 (25%) were found to have them. Of this group, seven (26%) leads were malfunctioning.
There were 65 Riata failures and 5 Riata ST failures, for a 6.2% incidence of all-cause failures. Of this group, 47 failures were due to electrical malfunctions, including seven from the externalized conductors contributing to the malfunction, as noted before. An additional 20 leads had externalized conductors with normal function, but met the study definition of failure.
In contrast, there were 23 lead failures among 1,668 patients with Quattro Secure leads (1.37%).
In univariate analysis, neither age, gender, primary or secondary indication for ICD implantation, ejection fraction, type of cardiac disease, nor lead placement location were significantly associated with risk for lead failure. The same was true when the Riata ST leads were subtracted from the equation.
Signs of failure included noise resulting in inappropriate therapy, elevated thresholds requiring lead replacement resulting in loss of capture and syncope, abnormal pacing impedance, abnormal high-voltage impedance, decline in R value requiring lead replacement, and failed defibrillation threshold testing with abnormal high-voltage impedance, requiring lead replacement.
Dr. Abdelhadi noted that the study was limited by its retrospective design, the fact that only 10% of leads were examined for externalized conductors, and the relatively short follow-up in the Riata ST group compared with the other two groups. In addition, most lead failures were not confirmed by returned-product analysis.
The study confirms what was already known and what many meeting attendees expected to hear, commented Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, in an interview.
"The bottom line is that it’s all about the patients, and how we take care of them, advise them, and whether we explant the leads. The whole international heart rhythm community is focused on this question and what to do so that there aren’t deaths that result from noise, that there aren’t deaths from unnecessary lead extractions," he said. Dr. Calkins was not involved in the study, but he moderated a media briefing where Dr. Abdelhadi presented the data.
Dr. Abdelhadi and Dr. Calkins reported having no current conflicts of interest to disclose.
BOSTON – An independent investigation by seven U.S. centers has shown that recently recalled Riata implantable cardioverter defibrillator leads are significantly more prone to failure than are leads made by a different manufacturer.
A retrospective study of all patients older than 18 years who received ICDs with either Riata or Riata ST leads (St. Jude Medical) showed a failure rate of 1.93% per patient-year for Riata leads, significantly more than the rate of 0.43% for the comparator lead, Quattro Secure (Medtronic) (P less than .0001). The failure rate for Riata ST leads was 0.50%, a nonsignificant difference, reported Dr. Raed Abdelhadi of the Minneapolis Heart Institute.
The Riata and Riata ST ICD leads are prone to inside-out and outside-in silicone insulation defects, which can cause lead malfunction and externalization (breach of the outer insulation, visible outside the lead body) of the conductor cables, but until this study, there were no independent, multicenter investigations into the rate of lead failure, failure signs, or clinical sequelae, he said.
"Without such data, it is not possible to design evidence-based management strategies or to advise Riata patients of their risks," he said at the annual meeting of the Heart Rhythm Society.
Investigators at the seven centers looked at all-cause failures and electrical malfunctions in 774 patients who received an 8-French Riata lead (mean follow-up, 4.2 years), and 307 patients who received a 7-French Riata ST lead (mean follow-up, 3.3 years). ICD leads with silicone-polyurethane copolymer insulation (RIATA ST Optim and Durata leads) were excluded.
For comparison, they obtained data on the Quattro Secure ICD leads from a three-center study of the longevity and risk factors for failure of Sprint Fidelis leads (Circulation 2011;123:358-63).
The investigators defined lead failure as either "abnormal impedance leading to replacement, electrical noise manifested by nonphysiologic signals, increase in pacing threshold or decline in R-wave amplitude necessitating lead placement, inability to provide effective therapy due to apparent lead abnormality, or externalized conductor, clearly demonstrated on fluoroscopy or x-ray ... even if the lead was functioning normally and electrically intact."
Neither functional abnormalities (for example, exit block and physiologic oversensing in the presence of an electrically intact lead) nor lead displacement (unless caused by a bad fixation mechanism) were considered to be failures.
Of the 1,081 Riata and Riata ST leads implanted, 712 (66%) were active and functioning normally at last follow-up. Of the 302 (28%) removals from service, 266 were owing to death, 10 to transplant, 5 to perforation, 4 to infection, and 17 to other causes.
In all, 10% of leads in the study were examined for externalized conductors, and 27 (25%) were found to have them. Of this group, seven (26%) leads were malfunctioning.
There were 65 Riata failures and 5 Riata ST failures, for a 6.2% incidence of all-cause failures. Of this group, 47 failures were due to electrical malfunctions, including seven from the externalized conductors contributing to the malfunction, as noted before. An additional 20 leads had externalized conductors with normal function, but met the study definition of failure.
In contrast, there were 23 lead failures among 1,668 patients with Quattro Secure leads (1.37%).
In univariate analysis, neither age, gender, primary or secondary indication for ICD implantation, ejection fraction, type of cardiac disease, nor lead placement location were significantly associated with risk for lead failure. The same was true when the Riata ST leads were subtracted from the equation.
Signs of failure included noise resulting in inappropriate therapy, elevated thresholds requiring lead replacement resulting in loss of capture and syncope, abnormal pacing impedance, abnormal high-voltage impedance, decline in R value requiring lead replacement, and failed defibrillation threshold testing with abnormal high-voltage impedance, requiring lead replacement.
Dr. Abdelhadi noted that the study was limited by its retrospective design, the fact that only 10% of leads were examined for externalized conductors, and the relatively short follow-up in the Riata ST group compared with the other two groups. In addition, most lead failures were not confirmed by returned-product analysis.
The study confirms what was already known and what many meeting attendees expected to hear, commented Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, in an interview.
"The bottom line is that it’s all about the patients, and how we take care of them, advise them, and whether we explant the leads. The whole international heart rhythm community is focused on this question and what to do so that there aren’t deaths that result from noise, that there aren’t deaths from unnecessary lead extractions," he said. Dr. Calkins was not involved in the study, but he moderated a media briefing where Dr. Abdelhadi presented the data.
Dr. Abdelhadi and Dr. Calkins reported having no current conflicts of interest to disclose.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Athletes With ICDs Have a Sporting Chance for Safe Play
BOSTON – Athletes with implantable cardioverter defibrillators may not need to be sidelined when the action gets fast and furious, say investigators in a multicenter study presented at the annual meeting of the Heart Rhythm Society.
Although international consensus statements caution against anything more vigorous than golf, bowling, or billiards for people with ICDs, an analysis of data from a prospective, multinational registry shows that although 10%* of athletes with ICDs received a shock from their devices during competition or practice, there were no serious adverse events, reported Dr. Rachel Lampert of the division of cardiology at Yale University, New Haven, Conn.
"Shocks were not rare during sports. However, no serious health consequences occurred. Most patients returned to sports having received shocks while playing, implying that the negative impact on quality of life of the shocks was offset for them on the quality of life from sports participation," Dr. Lampert said.
The findings suggest that the decision to return to a sport or quit it after an ICD implantation should be individualized, she added.
Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, said in an interview that he is comfortable with allowing many but not all patients with ICDs to return to an active sport.
"The one exception is arrhythmogenic right ventricular dysplasia, where exercise actually causes the disease to progress. That’s very different from the long QT or the Brugada syndrome, where exercise doesn’t have any impact on the underlying disease or disease progression," said Dr. Calkins, who was not involved in the study.
Dr. Lampert and her colleagues recruited 372* athletes aged 10-60 years (median, 33 years) with ICDs who participated in either competitive or dangerous sports (defined as any sport where sudden loss of control could cause injury).
The athletes were self-enrolled and volunteered to participate after learning of the study from patient-advocacy groups, mailing lists, or word of mouth. The investigators interviewed the athletes by telephone and obtained their medical records for capturing sports-related and clinical data, then followed up with phone calls every 6 months.
In all, 67% were male and 94% were white, with a mean time since initial ICD implantation of 27 months. The mean left ventricular ejection fraction was 60%. Nearly two-thirds (62%) were taking beta-blockers. The primary cardiac diagnoses included long QT syndrome, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and coronary artery disease.
Competition was at the elite amateur level, which the authors defined as interscholastic varsity or junior varsity, or regional or national competitions.
During the median 31 months of follow-up, two patients died: a 52 year-old cyclist with coronary artery disease died at his desk at work after receiving multiple shocks; and a 34-year-old volleyball/basketball player with familial cardiomyopathy who died during a hospitalization for heart failure. Additionally, 9 patients were lost to follow-up (all were confirmed still alive), 6 withdrew, and 4 developed worsening cardiac or medical conditions precluding sports, leaving 351* patients for the analysis.
There were no cases of the primary end point: either tachyarrhythmic death or externally resuscitated tachyarrhythmia during or after sports, or of injury due to arrhythmia or shock during sports.
Overall, 77 patients received at least one shock during the study, 37 during sports. Of this group, four stopped sports completely, and seven stopped one or more sports. Five patients stopped participating in at least one sport because of shocks they received at rest or at other times when playing a sport.
Seven patients had eight ventricular arrhythmias requiring multiple shocks to (two to six) to terminate the event, occurring in patients with catecholaminergic polymorphic ventricular tachycardia, idiopathic ventricular fibrillation, or coronary artery disease.
There were 13 definite lead malfunctions, defined as noise on a lead or change in pacing parameters with a visualized lead abnormality, and 14 probable lead malfunctions (change in pacing function only).
Freedom from lead malfunction at 5 years from the time of implant was 93%, and at 10 years was 84%.
Dr. Lampert acknowledged that the study was limited by lack of a control group; patient self-selection, which may have led to underrepresentation of athletes who have received ICDS; and limited follow-up.
Nonetheless, "these data do not support blanket restriction of athletes with ICDs from participating in sports," she concluded.
The investigator-initiated study was supported by Medtronic, St. Jude Medical, and Boston Scientific. Dr. Lampert disclosed receiving honoraria from two of the companies (not specified). Dr. Calkins has previously consulted for and received research support from Medtronic.
*Correction, 5/14/12: An earlier version of this article misstated the number of patients and thus the percentage of patients receiving shocks.
BOSTON – Athletes with implantable cardioverter defibrillators may not need to be sidelined when the action gets fast and furious, say investigators in a multicenter study presented at the annual meeting of the Heart Rhythm Society.
Although international consensus statements caution against anything more vigorous than golf, bowling, or billiards for people with ICDs, an analysis of data from a prospective, multinational registry shows that although 10%* of athletes with ICDs received a shock from their devices during competition or practice, there were no serious adverse events, reported Dr. Rachel Lampert of the division of cardiology at Yale University, New Haven, Conn.
"Shocks were not rare during sports. However, no serious health consequences occurred. Most patients returned to sports having received shocks while playing, implying that the negative impact on quality of life of the shocks was offset for them on the quality of life from sports participation," Dr. Lampert said.
The findings suggest that the decision to return to a sport or quit it after an ICD implantation should be individualized, she added.
Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, said in an interview that he is comfortable with allowing many but not all patients with ICDs to return to an active sport.
"The one exception is arrhythmogenic right ventricular dysplasia, where exercise actually causes the disease to progress. That’s very different from the long QT or the Brugada syndrome, where exercise doesn’t have any impact on the underlying disease or disease progression," said Dr. Calkins, who was not involved in the study.
Dr. Lampert and her colleagues recruited 372* athletes aged 10-60 years (median, 33 years) with ICDs who participated in either competitive or dangerous sports (defined as any sport where sudden loss of control could cause injury).
The athletes were self-enrolled and volunteered to participate after learning of the study from patient-advocacy groups, mailing lists, or word of mouth. The investigators interviewed the athletes by telephone and obtained their medical records for capturing sports-related and clinical data, then followed up with phone calls every 6 months.
In all, 67% were male and 94% were white, with a mean time since initial ICD implantation of 27 months. The mean left ventricular ejection fraction was 60%. Nearly two-thirds (62%) were taking beta-blockers. The primary cardiac diagnoses included long QT syndrome, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and coronary artery disease.
Competition was at the elite amateur level, which the authors defined as interscholastic varsity or junior varsity, or regional or national competitions.
During the median 31 months of follow-up, two patients died: a 52 year-old cyclist with coronary artery disease died at his desk at work after receiving multiple shocks; and a 34-year-old volleyball/basketball player with familial cardiomyopathy who died during a hospitalization for heart failure. Additionally, 9 patients were lost to follow-up (all were confirmed still alive), 6 withdrew, and 4 developed worsening cardiac or medical conditions precluding sports, leaving 351* patients for the analysis.
There were no cases of the primary end point: either tachyarrhythmic death or externally resuscitated tachyarrhythmia during or after sports, or of injury due to arrhythmia or shock during sports.
Overall, 77 patients received at least one shock during the study, 37 during sports. Of this group, four stopped sports completely, and seven stopped one or more sports. Five patients stopped participating in at least one sport because of shocks they received at rest or at other times when playing a sport.
Seven patients had eight ventricular arrhythmias requiring multiple shocks to (two to six) to terminate the event, occurring in patients with catecholaminergic polymorphic ventricular tachycardia, idiopathic ventricular fibrillation, or coronary artery disease.
There were 13 definite lead malfunctions, defined as noise on a lead or change in pacing parameters with a visualized lead abnormality, and 14 probable lead malfunctions (change in pacing function only).
Freedom from lead malfunction at 5 years from the time of implant was 93%, and at 10 years was 84%.
Dr. Lampert acknowledged that the study was limited by lack of a control group; patient self-selection, which may have led to underrepresentation of athletes who have received ICDS; and limited follow-up.
Nonetheless, "these data do not support blanket restriction of athletes with ICDs from participating in sports," she concluded.
The investigator-initiated study was supported by Medtronic, St. Jude Medical, and Boston Scientific. Dr. Lampert disclosed receiving honoraria from two of the companies (not specified). Dr. Calkins has previously consulted for and received research support from Medtronic.
*Correction, 5/14/12: An earlier version of this article misstated the number of patients and thus the percentage of patients receiving shocks.
BOSTON – Athletes with implantable cardioverter defibrillators may not need to be sidelined when the action gets fast and furious, say investigators in a multicenter study presented at the annual meeting of the Heart Rhythm Society.
Although international consensus statements caution against anything more vigorous than golf, bowling, or billiards for people with ICDs, an analysis of data from a prospective, multinational registry shows that although 10%* of athletes with ICDs received a shock from their devices during competition or practice, there were no serious adverse events, reported Dr. Rachel Lampert of the division of cardiology at Yale University, New Haven, Conn.
"Shocks were not rare during sports. However, no serious health consequences occurred. Most patients returned to sports having received shocks while playing, implying that the negative impact on quality of life of the shocks was offset for them on the quality of life from sports participation," Dr. Lampert said.
The findings suggest that the decision to return to a sport or quit it after an ICD implantation should be individualized, she added.
Dr. Hugh Calkins, professor of cardiology at the Johns Hopkins Heart and Vascular Institute, Baltimore, and vice president of the Heart Rhythm Society, said in an interview that he is comfortable with allowing many but not all patients with ICDs to return to an active sport.
"The one exception is arrhythmogenic right ventricular dysplasia, where exercise actually causes the disease to progress. That’s very different from the long QT or the Brugada syndrome, where exercise doesn’t have any impact on the underlying disease or disease progression," said Dr. Calkins, who was not involved in the study.
Dr. Lampert and her colleagues recruited 372* athletes aged 10-60 years (median, 33 years) with ICDs who participated in either competitive or dangerous sports (defined as any sport where sudden loss of control could cause injury).
The athletes were self-enrolled and volunteered to participate after learning of the study from patient-advocacy groups, mailing lists, or word of mouth. The investigators interviewed the athletes by telephone and obtained their medical records for capturing sports-related and clinical data, then followed up with phone calls every 6 months.
In all, 67% were male and 94% were white, with a mean time since initial ICD implantation of 27 months. The mean left ventricular ejection fraction was 60%. Nearly two-thirds (62%) were taking beta-blockers. The primary cardiac diagnoses included long QT syndrome, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and coronary artery disease.
Competition was at the elite amateur level, which the authors defined as interscholastic varsity or junior varsity, or regional or national competitions.
During the median 31 months of follow-up, two patients died: a 52 year-old cyclist with coronary artery disease died at his desk at work after receiving multiple shocks; and a 34-year-old volleyball/basketball player with familial cardiomyopathy who died during a hospitalization for heart failure. Additionally, 9 patients were lost to follow-up (all were confirmed still alive), 6 withdrew, and 4 developed worsening cardiac or medical conditions precluding sports, leaving 351* patients for the analysis.
There were no cases of the primary end point: either tachyarrhythmic death or externally resuscitated tachyarrhythmia during or after sports, or of injury due to arrhythmia or shock during sports.
Overall, 77 patients received at least one shock during the study, 37 during sports. Of this group, four stopped sports completely, and seven stopped one or more sports. Five patients stopped participating in at least one sport because of shocks they received at rest or at other times when playing a sport.
Seven patients had eight ventricular arrhythmias requiring multiple shocks to (two to six) to terminate the event, occurring in patients with catecholaminergic polymorphic ventricular tachycardia, idiopathic ventricular fibrillation, or coronary artery disease.
There were 13 definite lead malfunctions, defined as noise on a lead or change in pacing parameters with a visualized lead abnormality, and 14 probable lead malfunctions (change in pacing function only).
Freedom from lead malfunction at 5 years from the time of implant was 93%, and at 10 years was 84%.
Dr. Lampert acknowledged that the study was limited by lack of a control group; patient self-selection, which may have led to underrepresentation of athletes who have received ICDS; and limited follow-up.
Nonetheless, "these data do not support blanket restriction of athletes with ICDs from participating in sports," she concluded.
The investigator-initiated study was supported by Medtronic, St. Jude Medical, and Boston Scientific. Dr. Lampert disclosed receiving honoraria from two of the companies (not specified). Dr. Calkins has previously consulted for and received research support from Medtronic.
*Correction, 5/14/12: An earlier version of this article misstated the number of patients and thus the percentage of patients receiving shocks.
FROM THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: While 9% of athletes with ICDs received a shock from their devices during competition or practice, there were no serious adverse events.
Data Source: Data were taken from a prospective registry study.
Disclosures: The investigator-initiated study was supported by Medtronic, St. Jude Medical, and Boston Scientific. Dr. Lampert disclosed receiving honoraria from two of the companies (not specified). Dr. Calkins has previously consulted for and received research support from Medtronic.