User login
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
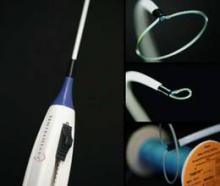
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
BOSTON – A percutaneous device that snares the left atrial appendage with a lariatlike suture was successful at closing the appendage in a majority of cases in a pilot study, reported an investigator at the annual meeting of the Heart Rhythm Society.
In 82 of 89 patients (92%) with atrial fibrillation (AF) who were ineligible for warfarin, SentreHeart Inc.’s Lariat suture delivery device successfully closed the left atrial appendage (LAA) down to a diameter of 1 mm or less, reported Dr. Randall Lee, professor of medicine in the division of cardiology at the University of California, San Francisco.
Among 65 patients available for follow-up, 64 (98%) still had complete closure of the LAA (defined as a gap of 1 mm or less), and 1 patient (2%) had a gap smaller than 2 mm.
The device "can be considered an option for high-risk patients with atrial fibrillation who are at high risk for embolic stroke and have contraindications or intolerance to anticoagulation," said Dr. Lee, who is a consultant to and has an equity stake in the company developing the device.
Dr. Richard I. Fogel, who was not involved in the study, called these early results "very exciting," and noted that with AF, the prime directive is to protect the brain.
"Some patients just can’t tolerate any anticoagulation for various reasons, such as the risk of falls or blood dyscrasias, and now that we have other technologies to prevent their stroke risk, I think it’s very good for the field and for our patients," he said in an interview. Dr. Fogel moderated the session at which these data were presented.
Unlike Atritech’s Watchman atrial closure device, which is placed percutaneously into the ostium of the LAA to trap emboli, the Lariat device is a percutaneous adaptation of older, open-chest suture ligation techniques.
The device is inserted in a procedure involving a transseptal catheter and pericardial access catheter with magnetic guide wires, one placed inside and one outside of the appendage to stabilize it. The transseptal catheter contains an occlusion balloon that helps to identify the appendage on imaging. The Lariat contains a preloaded suture loop that is placed via an over-the-wire approach onto the appendage, and the appendage is ligated.
The investigators evaluated the device in a nonrandomized, single center study of 119 patients with AF and a CHADS2 score greater than 1 who were ineligible for anticoagulation with warfarin. The patients were aged 18 years or older and had nonvalvular AF; all had a life expectancy of at least 1 year.
Patients with LAA width greater than 40 mm were excluded, as were those in whom the LAA anatomy might complicate a percutaneous approach, including those with a superiorly oriented LAA with the apex directed behind the pulmonary trunk, and patients with bi- or multilobed LAAs in which the lobes were oriented in different planes exceeding 40 mm.
Of the 119 screened, 103 were deemed to be eligible for the procedure, and 14 were excluded by imaging before the procedure because of adhesions (3 patients) or mobile thrombi (11 patients).
Of the 89 remaining patients, the procedure was deemed to be successful in 85 (95.5%).
The procedure failed in four patients (4.5%). Causes of failure were pericardial effusion in two patients, anatomical contraindication to insertion of the transseptal catheter in one patient, and failure to capture the appendage because of adhesions in one patient.
No patient experienced a loss of appendage closure or capture at either the 1-day or 30-day follow-up, as determined by angiography and transesophageal echocardiography. At 90 days, the closure/capture integrity was still good among all 81 patients available for follow-up. (Four refused follow-up beyond 60 days.)
Adverse events included access-related complications in 3 patients; chest pain from a pig tail catheter left in place for 24 hours following the procedure in 20 patients; pericarditis in 2 patients; late pericardial effusion in 1 patient; one hemorrhagic and one lacunar stroke, each occurring more than 6 months after the procedure; and two deaths more than 6 months after the procedure, both unrelated to the procedure.
The investigators are planning a prospective, adjudicated, multicenter study to more objectively evaluate the device, Dr. Lee said.
The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.
AT THE ANNUAL MEETING OF THE HEART RHYTHM SOCIETY
Major Finding: In 82 of 89 patients (92%), the Lariat suture delivery device successfully closed the left atrial appendage down to a diameter of 1 mm or less.
Data Source: Data are from a single-center, nonrandomized study of 89 AF patients who were ineligible for warfarin.
Disclosures: The study was supported by SentreHeart Inc. Dr. Lee is a consultant to the company and owns stock in it. Dr. Fogel disclosed that he has received grants for clinical research and grants for educational activities from St. Jude Medical, Medtronic, and Guidant, and owns stock in Medtronic and Guidant.