User login
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
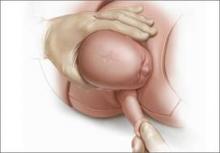
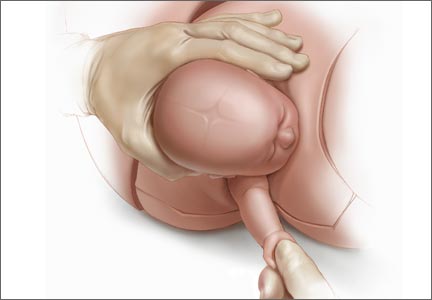
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.