User login
Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
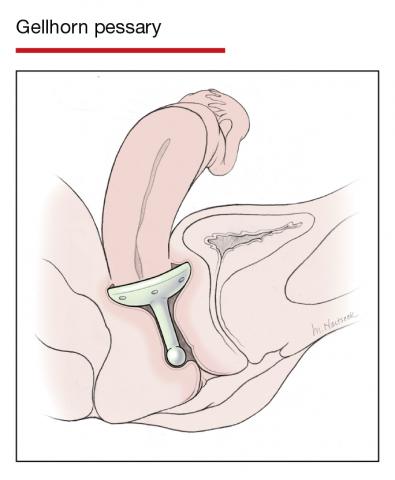
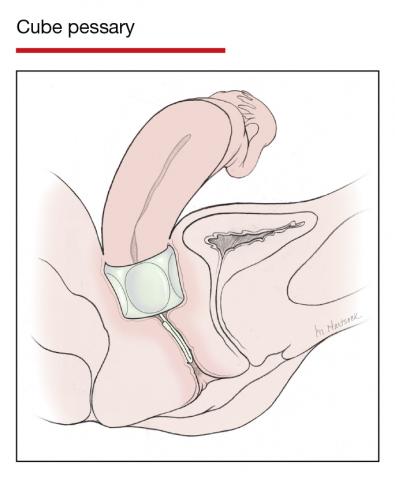
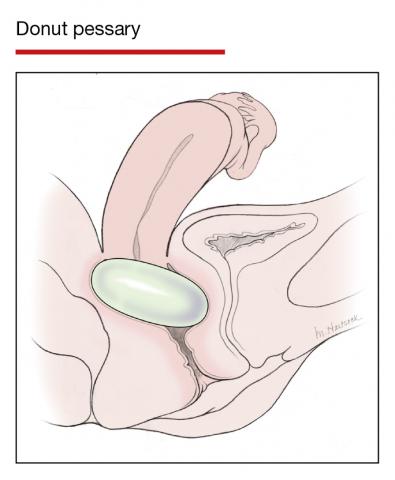
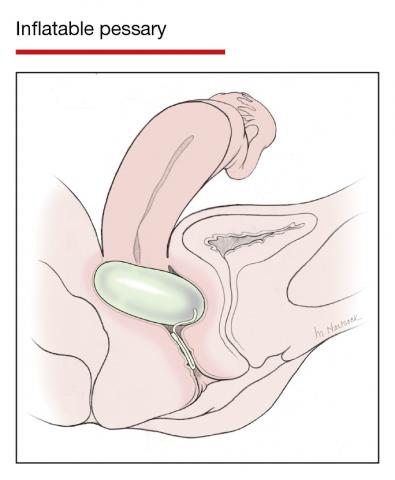
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13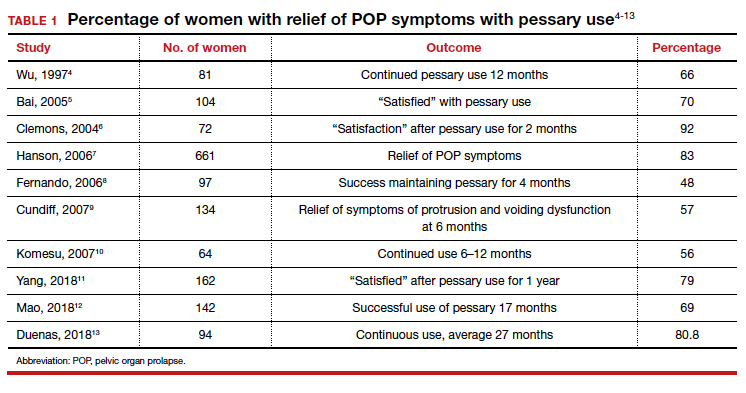
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17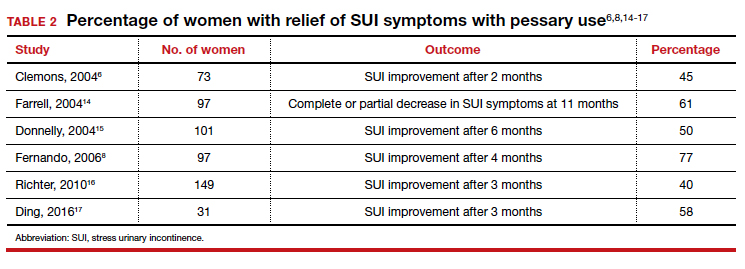
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
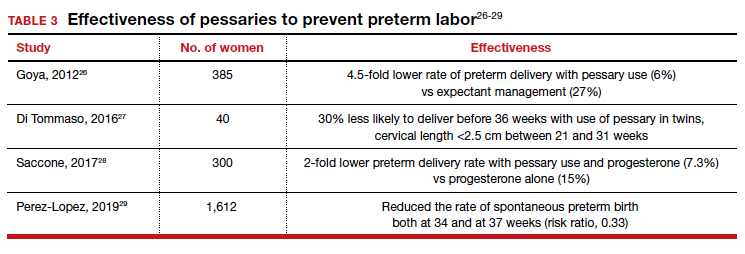
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
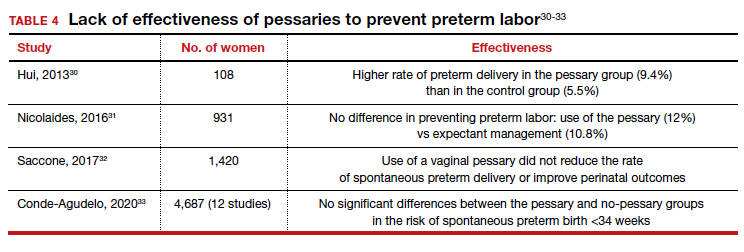
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
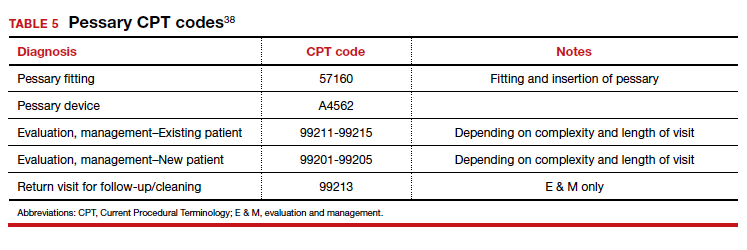
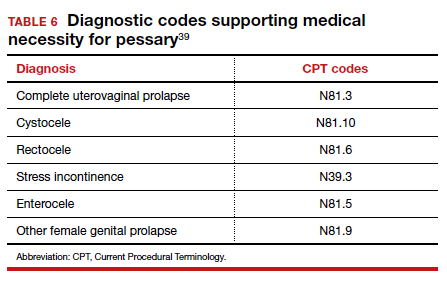
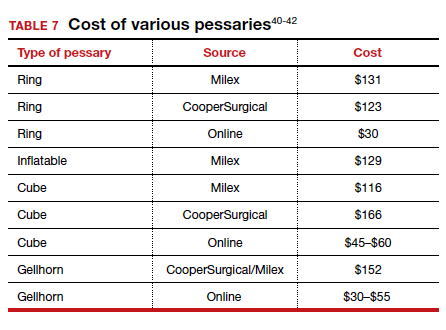
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
Pessaries for POP and SUI: Your options and guidance on use
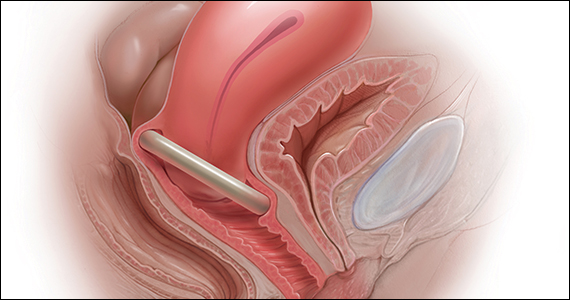
Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
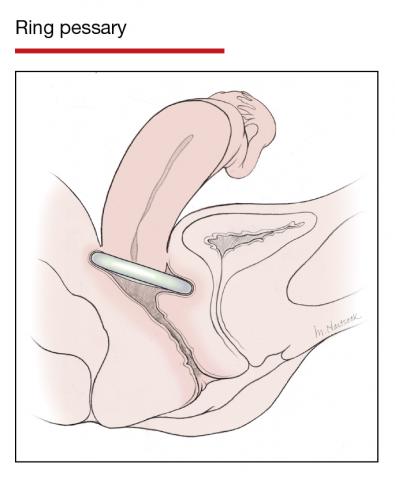
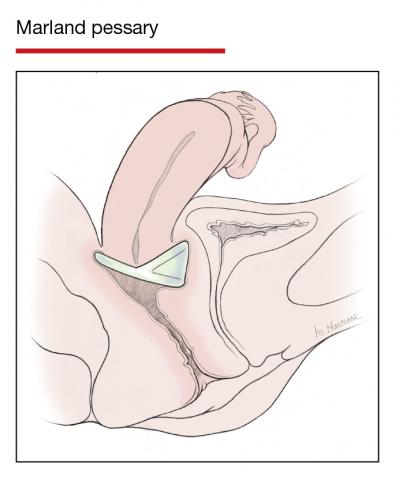
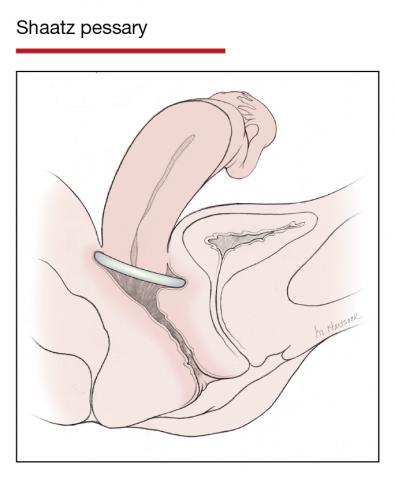
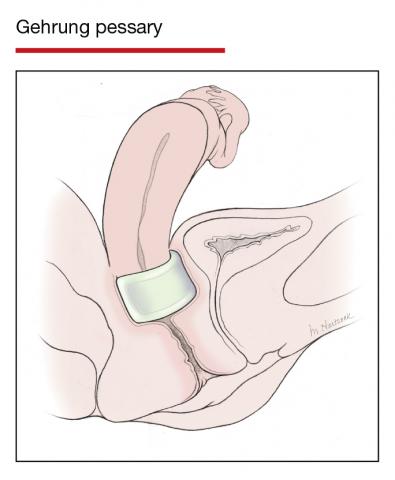
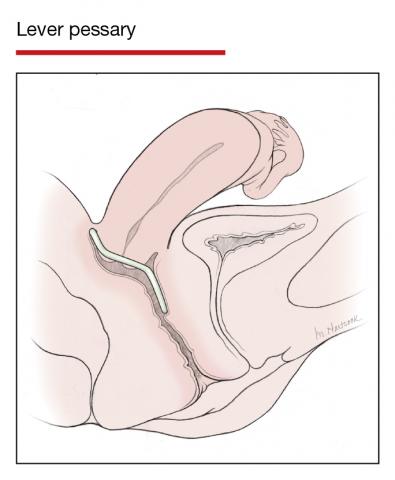
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.
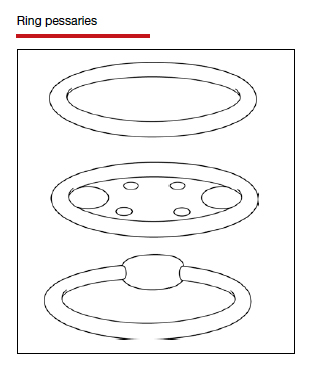
The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11
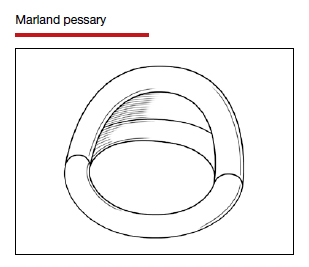
The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.
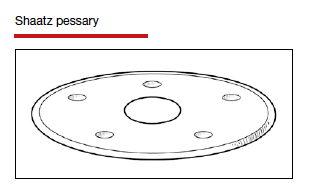
The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.
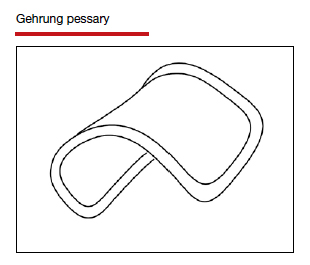
Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.
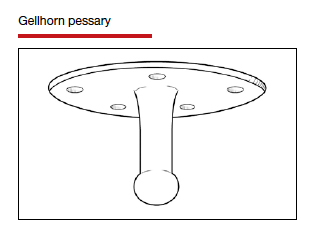
The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.
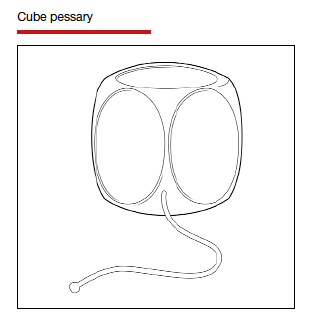
The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).
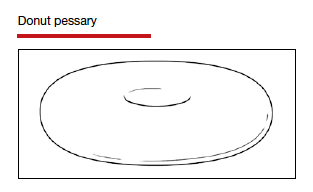
The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.
Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 2: Pathophysiology and causation
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 1: Can it be predicted?
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Eight tools for improving obstetric patient safety and unit performance
Obstetricians, obstetric nurses, nurse managers, and obstetric department heads are almost always well-trained, hard working, highly motivated individuals dedicated to providing the best possible care for their patients. Nevertheless, errors in the provision of care are all too common.1–3 Even though these errors are confined to a small percentage of patient interactions, they engender profound consequences: injuries to mothers or their babies, higher costs to treat associated complications, and medical-legal suits that can entangle both clinicians and plaintiffs for years.
Why do such errors occur when it is the goal of well-trained and dedicated practi-tioners to provide error-free care? There are several reasons:
- The provision of medical care in the early 21st Century is an enormously complex endeavor.
- Physicians and nurses are human beings and, therefore, do not—and never will—perform perfectly all the time, in every situation, with every patient.
- The systems within which care providers work and the tools with which they work are often suboptimal and inefficient and are not designed to maximize patient safety.
- Financial constraints on hospital systems and physician practices dictate that obstetricians and obstetric nurses care for as many patients as possible in limited periods of time.
How then can obstetrics professionals seek to eradicate or at least decrease the number of medical errors that occur during the provision of maternity care?
To accomplish this, we must address the core issues at the root of these medical errors. Solutions must be implemented to 1) simplify the often unnecessary complexity of delivering medical care and 2) create systems and tools that minimize errors and catch those that do occur before they can cause harm.
Yet, how is this to be accomplished? In this article, I describe eight tools developed over time by clinicians who have worked in the field of obstetric patient safety. These tools provide some answers and concrete starting points.
TOOL 1: CONTINUING EDUCATION
William Osler once said, “It is astonishing with how little reading a doctor can practice medicine, but it is not astonishing how badly he may do it.”
As the years out of residency and nursing school accumulate, clinicians—both obstetricians and obstetric nurses—find it all too easy to continue to practice pretty much the way they did during training. However, medical science changes, new protocols improve on the old, and new techniques and medications are introduced yearly into the practice arena. If a clinician is to deliver the best possible care, he or she has to keep abreast of these developments in obstetrics and refresh his or her memory from time to time about things learned long ago. Such acquisition of new and review of old obstetric knowledge can be achieved only through ongoing study.
There are many ways continuing education can be accomplished. You can read new editions of textbooks when they are published or follow an obstetric journal through its yearly cycle. Cutting-edge, clinically oriented, interactive courses in all major areas of obstetrics are available to clinicians online. The recertification criteria of the American College of Obstetricians and Gynecologists (ACOG), state licensing requirements, and individual obstetric department recredentialing requirements often mandate such continuing education.
TOOL 2: SIMULATION PROGRAMS
Most obstetric emergencies, especially the most dangerous ones, occur infrequently, making it difficult for the many members of any labor and delivery unit to have their skills sharply honed to best deal with them. This is less of a problem at busy institutions where, simply due to the numbers of patients cared for, such emergencies are encountered on a regular basis. But at smaller facilities they are, fortunately, rare. The only way a unit can maintain its competency to handle such situations when they do arise—and they will—is to practice them in simulation mode.
There is now an increasing amount of literature demonstrating that simulation programs are effective not only at improving the knowledge base of obstetrics providers but also at improving Apgar scores, reducing admissions to neonatal intensive care units (NICUs), and preventing brachial plexus injuries.4
An effective simulation program should contain the following features:
- a thorough, didactic review of the clinical aspects of emergency care for all of the major obstetric emergencies (postpartum hemorrhage, shoulder dystocia, eclamptic seizure, maternal collapse, and urgent cesarean section)
- practice drills for the above
- training in teamwork and communication skills
- frequent repetition, ideally with each major obstetric emergency being covered twice per year.
Many institutions have developed simulation training centers. While these can be excellent teaching facilities, something is lost if simulation training is not done on the actual unit where obstetricians and obstetric nurses will encounter emergencies. Simulation programs also should be time-efficient and should be scheduled to make it easy for obstetrics personnel to participate. For greater convenience and knowledge retention, it is better to have short simulation programs at frequent intervals than day-long programs once per year or every other year.
Related Article: How simulation can train, and refresh, physicians for critical OB events Robert Gherman, MD; Andrew Satin, MD; Roxane Gardner, MD, MPH
TOOL 3: INTERNAL AUDITS
It is a mantra in business that you can’t fix what you can’t measure. And while obstetric units usually keep track of such things as rates of cesarean section, elective induction at less than 39 weeks, and admission to the NICU, it is rare that data are kept on other extremely important information. For instance, how often is an induction started with no indication for it written in the admission note? How often is the vacuum or forceps applied with no note documenting the reason or the discussion of risks and benefits with the patient? How often does estimated fetal weight go unnoted in the medical record of a mother with gestational diabetes?
An audit program, either in computer format or with manual collection on paper, is a vital tool for each labor and delivery unit to use in assessing the quality of the care it provides. Such an audit, by covering a sufficiently large number of clinical data points, can give tremendous insight into the specifics of the unit’s performance over the range of obstetric care situations. It will show where things are being done well and where they are not. The audit becomes even more valuable if it is designed so that each of the measured data points can be evaluated for individual clinician performance as well as for the labor and delivery unit as a whole.
Similar audits also should be conducted in individual physician offices and obstetric clinics. Many of the errors that occur in providing obstetric care occur prenatally: tests not performed, lack of follow-up of known problems, or poor communication with patients or with the labor and delivery unit.
One of the major benefits of audit programs that are conducted on a regular basis—every 6 months or annually are common intervals—is that trends in performance in each area of care can be evaluated. As deficiencies are pointed out to providers, their compliance with best care practices should improve from cycle to cycle.
TOOL 4: BEST PRACTICE PROTOCOLS
Medicine is now well past the point where protocols are seen as “restrictive” or “advocating cookbook medicine.” Well-designed protocols summarize best practices derived from evidence-based studies and the consensus of obstetric experts. They serve as convenient reminders to physicians in various clinical situations so that these clinicians do not have to rely solely on what they happen to remember about caring for a given condition. Protocols also provide a certain uniformity of care, which in itself decreases the likelihood of errors being made.
Each obstetric department should have a set of protocols to cover the most common obstetric situations, such as:
- premature rupture of membranes
- instrumental vaginal deliveries
- oxytocin administration.
Each unit does not have to devise its own protocols; ACOG and nearby academic institutions are excellent sources for protocols that can be replicated and implemented so that they do not have to be created de novo.
Related Article: More strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD; Alexis C. Gimovsky, MD (January 2011)
TOOL 5: SAFETY CHECKLISTS
Just as well-designed protocols can serve as convenient reminders of best practices, low-tech physical checklists can be kept at nursing stations and in labor and delivery rooms to serve as reminders of best practices during obstetric emergencies. For instance, having a laminated set of easy-to-read protocols for postpartum hemorrhage, eclamptic seizure, maternal collapse, and shoulder dystocia in a delivery room can allow a charge nurse or other supervisor to check to make sure all proper procedures are being performed by the team actually administering care to a patient in crisis, with nothing important overlooked.
Related Article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations Robert L. Barbieri, MD (Editorial, August 2013)
TOOL 6: COMPLETE DOCUMENTATION
Almost as many lawsuits are lost because of poor documentation as are lost because of inappropriate medical care. The obstetric literature,1 and my own experience with the medical-legal system, clearly demonstrate the need for appropriate, careful documentation of the events that transpire during patient care. Notes do not have to be especially long or verbose—but they must contain all relevant information and describe the obstetrician’s thinking at various decision points.
Documentation can be inadequate because of time constraints, poor understanding of the events that transpired, or simply a lack of remembering to include salient points that should be covered in a clinical note.
Clinicians can be prompted to include key aspects of care in the medical record by using prepared templates. Such templates are easy to fill out, remind clinicians to document information that would otherwise not get recorded, and result in a much more complete patient chart. By using a template, a clinician would never forget to record the head-to-body delivery interval after a shoulder dystocia or whether a fetal heart rate was obtained in the operating room just prior to starting a cesarean section.
Related Article: Sound strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD (December 2010)
TOOL 7: SMART MEDICAL RECORDS
In obstetrics we are fortunate that there is a limited range of issues that recur repeatedly, such as gestational hypertension, placental abruption, and fetal distress. One soon gains experience in managing these conditions and, with the help of best-practice protocols, optimal care almost always can be provided.
Still, many clinical presentations can pose diagnostic challenges, especially in atypical cases. Moreover, clinicians managing a patient’s care may not immediately remember the best means of evaluating and treating a certain condition in specific circumstances. For example, at 3:00 am it may be difficult to recall whether it is nifedipine or labetalol that should be avoided with asthmatic patients or which antibiotic formulation is currently recommended for prophylaxis in a patient with premature rupture of membranes at 30 weeks’ gestation who is allergic to penicillin.
Smart medical records, already widely used in other fields of medicine, are an antidote to this problem. When certain diagnoses, physical findings, clinical details, or laboratory data are entered into specific fields in an electronic medical record, templates that have been added to the record automatically appear to show relevant information, such as tests that should be performed, treatments that should be administered, and alternative diagnoses that should be considered. Such reminders are not presented as obligations or “hard stops”; they are usually displayed in the form of easily dismissible pop-ups or “reminder bubbles” that appear on the screen and serve solely to jog memory and provide information.
Such smart electronic medical record features can be provided either by the main electronic medical record vendor or added as subprograms by other providers.
Related Article: EHRs and medicolegal risk: How they help, when they could hurt Martin L. Gimovsky, MD; Baohuong N. Trans, DO (March 2013)
TOOL 8: MATERNITY UNIT ON-SITE CONSULTATIONS
Every labor and delivery unit has its own culture, a combination of institutional history and the personality of the doctors and nurses working there. Some units function efficiently, have the most modern equipment, and provide superb medical care. Other units have less than adequate facilities, remain entrenched in older practices, and have disruptive or uncooperative personnel that interfere with the smooth running of the unit. Moreover, each maternity unit, based on its resources, patient population, and staff skills, devises its own solutions to the same sorts of problems that all other obstetric units share. Unfortunately, there is little collaboration between units to discuss common problems and trade best practices. The result is that all too often each unit invents its own “wheel” when many excellent “wheels” already have been developed for the same issues around the country.
An on-site visit by an outside consultant—an obstetrician, an obstetric nurse, or both—can identify ongoing institutional problems, point out care deficiencies the unit may not be aware of, and provide resources and ideas to help solve the issues identified. Moreover, an outside consultant can offer unbiased and authoritative opinions to help move initiatives that may be stalled by local personalities or institutional politics.
Some features that a well-conducted on-site consultation will evaluate are:
- adequacy of obstetric triage
- capacity to perform stat cesarean sections 24/7
- 24-hour availability of obstetricians, anesthesiologists, pediatricians, and operating room teams
- preparation for handling various obstetric emergencies
- oxytocin administration protocols and compliance
- adequacy of physician and nurse charting
- ongoing skills assessment of fetal heart-rate monitor interpretation
- presence of practitioners whose disruptive behavior compromises the safety of the unit
- preparation for nonmedical emergencies, such as infant abduction, natural disaster; fire; shooter; or disruptive patients, visitors, or staff.
IMPLEMENTATION CAN EQUAL SAFER CARE
As long as people have babies, less than desirable outcomes will occasionally occur. As long as care providers are human beings, the provision of obstetric care will continue to be imperfect.
It is up to those entrusted with the responsibility of caring for mothers and their babies to provide as much support and backup as possible to obstetricians and obstetric nurses, all of whom sincerely desire to do everything possible to deliver safe care to their patients.
Tools for providing such support and backup are available and can be implemented fairly easily on most obstetric units. They do involve an expenditure of both time and money. However, the most important requirement for success is an institutional willingness to 1) acknowledge that the care a given unit provides can be improved, 2) perform an in-depth evaluation of the quality of care currently being administered, and 3) move ahead with the sorts of tools discussed in this article that will enable clinicians to provide optimal care for mothers and babies.
SHARE YOUR EXPERIENCE!
Did implementation of a tool described in this article solve a problem or improve performance for your obstetric unit? Tell us about it by emailing to: obg@frontlinemedcom.com Please include your name, city, and state.
- Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279–1283.
- Gluck PA. Medical error theory. Obstet Gynecol Clin North Am. 2008;35(1):11–17, vii.
- Anakiraman V, Ecker J. Quality in obstetric care: measuring what matters. Obstet Gynecol. 2010;116(3):728–732.
- Draycott T, Sibanda T, Owen L, et al. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113(2):177–182.
Obstetricians, obstetric nurses, nurse managers, and obstetric department heads are almost always well-trained, hard working, highly motivated individuals dedicated to providing the best possible care for their patients. Nevertheless, errors in the provision of care are all too common.1–3 Even though these errors are confined to a small percentage of patient interactions, they engender profound consequences: injuries to mothers or their babies, higher costs to treat associated complications, and medical-legal suits that can entangle both clinicians and plaintiffs for years.
Why do such errors occur when it is the goal of well-trained and dedicated practi-tioners to provide error-free care? There are several reasons:
- The provision of medical care in the early 21st Century is an enormously complex endeavor.
- Physicians and nurses are human beings and, therefore, do not—and never will—perform perfectly all the time, in every situation, with every patient.
- The systems within which care providers work and the tools with which they work are often suboptimal and inefficient and are not designed to maximize patient safety.
- Financial constraints on hospital systems and physician practices dictate that obstetricians and obstetric nurses care for as many patients as possible in limited periods of time.
How then can obstetrics professionals seek to eradicate or at least decrease the number of medical errors that occur during the provision of maternity care?
To accomplish this, we must address the core issues at the root of these medical errors. Solutions must be implemented to 1) simplify the often unnecessary complexity of delivering medical care and 2) create systems and tools that minimize errors and catch those that do occur before they can cause harm.
Yet, how is this to be accomplished? In this article, I describe eight tools developed over time by clinicians who have worked in the field of obstetric patient safety. These tools provide some answers and concrete starting points.
TOOL 1: CONTINUING EDUCATION
William Osler once said, “It is astonishing with how little reading a doctor can practice medicine, but it is not astonishing how badly he may do it.”
As the years out of residency and nursing school accumulate, clinicians—both obstetricians and obstetric nurses—find it all too easy to continue to practice pretty much the way they did during training. However, medical science changes, new protocols improve on the old, and new techniques and medications are introduced yearly into the practice arena. If a clinician is to deliver the best possible care, he or she has to keep abreast of these developments in obstetrics and refresh his or her memory from time to time about things learned long ago. Such acquisition of new and review of old obstetric knowledge can be achieved only through ongoing study.
There are many ways continuing education can be accomplished. You can read new editions of textbooks when they are published or follow an obstetric journal through its yearly cycle. Cutting-edge, clinically oriented, interactive courses in all major areas of obstetrics are available to clinicians online. The recertification criteria of the American College of Obstetricians and Gynecologists (ACOG), state licensing requirements, and individual obstetric department recredentialing requirements often mandate such continuing education.
TOOL 2: SIMULATION PROGRAMS
Most obstetric emergencies, especially the most dangerous ones, occur infrequently, making it difficult for the many members of any labor and delivery unit to have their skills sharply honed to best deal with them. This is less of a problem at busy institutions where, simply due to the numbers of patients cared for, such emergencies are encountered on a regular basis. But at smaller facilities they are, fortunately, rare. The only way a unit can maintain its competency to handle such situations when they do arise—and they will—is to practice them in simulation mode.
There is now an increasing amount of literature demonstrating that simulation programs are effective not only at improving the knowledge base of obstetrics providers but also at improving Apgar scores, reducing admissions to neonatal intensive care units (NICUs), and preventing brachial plexus injuries.4
An effective simulation program should contain the following features:
- a thorough, didactic review of the clinical aspects of emergency care for all of the major obstetric emergencies (postpartum hemorrhage, shoulder dystocia, eclamptic seizure, maternal collapse, and urgent cesarean section)
- practice drills for the above
- training in teamwork and communication skills
- frequent repetition, ideally with each major obstetric emergency being covered twice per year.
Many institutions have developed simulation training centers. While these can be excellent teaching facilities, something is lost if simulation training is not done on the actual unit where obstetricians and obstetric nurses will encounter emergencies. Simulation programs also should be time-efficient and should be scheduled to make it easy for obstetrics personnel to participate. For greater convenience and knowledge retention, it is better to have short simulation programs at frequent intervals than day-long programs once per year or every other year.
Related Article: How simulation can train, and refresh, physicians for critical OB events Robert Gherman, MD; Andrew Satin, MD; Roxane Gardner, MD, MPH
TOOL 3: INTERNAL AUDITS
It is a mantra in business that you can’t fix what you can’t measure. And while obstetric units usually keep track of such things as rates of cesarean section, elective induction at less than 39 weeks, and admission to the NICU, it is rare that data are kept on other extremely important information. For instance, how often is an induction started with no indication for it written in the admission note? How often is the vacuum or forceps applied with no note documenting the reason or the discussion of risks and benefits with the patient? How often does estimated fetal weight go unnoted in the medical record of a mother with gestational diabetes?
An audit program, either in computer format or with manual collection on paper, is a vital tool for each labor and delivery unit to use in assessing the quality of the care it provides. Such an audit, by covering a sufficiently large number of clinical data points, can give tremendous insight into the specifics of the unit’s performance over the range of obstetric care situations. It will show where things are being done well and where they are not. The audit becomes even more valuable if it is designed so that each of the measured data points can be evaluated for individual clinician performance as well as for the labor and delivery unit as a whole.
Similar audits also should be conducted in individual physician offices and obstetric clinics. Many of the errors that occur in providing obstetric care occur prenatally: tests not performed, lack of follow-up of known problems, or poor communication with patients or with the labor and delivery unit.
One of the major benefits of audit programs that are conducted on a regular basis—every 6 months or annually are common intervals—is that trends in performance in each area of care can be evaluated. As deficiencies are pointed out to providers, their compliance with best care practices should improve from cycle to cycle.
TOOL 4: BEST PRACTICE PROTOCOLS
Medicine is now well past the point where protocols are seen as “restrictive” or “advocating cookbook medicine.” Well-designed protocols summarize best practices derived from evidence-based studies and the consensus of obstetric experts. They serve as convenient reminders to physicians in various clinical situations so that these clinicians do not have to rely solely on what they happen to remember about caring for a given condition. Protocols also provide a certain uniformity of care, which in itself decreases the likelihood of errors being made.
Each obstetric department should have a set of protocols to cover the most common obstetric situations, such as:
- premature rupture of membranes
- instrumental vaginal deliveries
- oxytocin administration.
Each unit does not have to devise its own protocols; ACOG and nearby academic institutions are excellent sources for protocols that can be replicated and implemented so that they do not have to be created de novo.
Related Article: More strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD; Alexis C. Gimovsky, MD (January 2011)
TOOL 5: SAFETY CHECKLISTS
Just as well-designed protocols can serve as convenient reminders of best practices, low-tech physical checklists can be kept at nursing stations and in labor and delivery rooms to serve as reminders of best practices during obstetric emergencies. For instance, having a laminated set of easy-to-read protocols for postpartum hemorrhage, eclamptic seizure, maternal collapse, and shoulder dystocia in a delivery room can allow a charge nurse or other supervisor to check to make sure all proper procedures are being performed by the team actually administering care to a patient in crisis, with nothing important overlooked.
Related Article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations Robert L. Barbieri, MD (Editorial, August 2013)
TOOL 6: COMPLETE DOCUMENTATION
Almost as many lawsuits are lost because of poor documentation as are lost because of inappropriate medical care. The obstetric literature,1 and my own experience with the medical-legal system, clearly demonstrate the need for appropriate, careful documentation of the events that transpire during patient care. Notes do not have to be especially long or verbose—but they must contain all relevant information and describe the obstetrician’s thinking at various decision points.
Documentation can be inadequate because of time constraints, poor understanding of the events that transpired, or simply a lack of remembering to include salient points that should be covered in a clinical note.
Clinicians can be prompted to include key aspects of care in the medical record by using prepared templates. Such templates are easy to fill out, remind clinicians to document information that would otherwise not get recorded, and result in a much more complete patient chart. By using a template, a clinician would never forget to record the head-to-body delivery interval after a shoulder dystocia or whether a fetal heart rate was obtained in the operating room just prior to starting a cesarean section.
Related Article: Sound strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD (December 2010)
TOOL 7: SMART MEDICAL RECORDS
In obstetrics we are fortunate that there is a limited range of issues that recur repeatedly, such as gestational hypertension, placental abruption, and fetal distress. One soon gains experience in managing these conditions and, with the help of best-practice protocols, optimal care almost always can be provided.
Still, many clinical presentations can pose diagnostic challenges, especially in atypical cases. Moreover, clinicians managing a patient’s care may not immediately remember the best means of evaluating and treating a certain condition in specific circumstances. For example, at 3:00 am it may be difficult to recall whether it is nifedipine or labetalol that should be avoided with asthmatic patients or which antibiotic formulation is currently recommended for prophylaxis in a patient with premature rupture of membranes at 30 weeks’ gestation who is allergic to penicillin.
Smart medical records, already widely used in other fields of medicine, are an antidote to this problem. When certain diagnoses, physical findings, clinical details, or laboratory data are entered into specific fields in an electronic medical record, templates that have been added to the record automatically appear to show relevant information, such as tests that should be performed, treatments that should be administered, and alternative diagnoses that should be considered. Such reminders are not presented as obligations or “hard stops”; they are usually displayed in the form of easily dismissible pop-ups or “reminder bubbles” that appear on the screen and serve solely to jog memory and provide information.
Such smart electronic medical record features can be provided either by the main electronic medical record vendor or added as subprograms by other providers.
Related Article: EHRs and medicolegal risk: How they help, when they could hurt Martin L. Gimovsky, MD; Baohuong N. Trans, DO (March 2013)
TOOL 8: MATERNITY UNIT ON-SITE CONSULTATIONS
Every labor and delivery unit has its own culture, a combination of institutional history and the personality of the doctors and nurses working there. Some units function efficiently, have the most modern equipment, and provide superb medical care. Other units have less than adequate facilities, remain entrenched in older practices, and have disruptive or uncooperative personnel that interfere with the smooth running of the unit. Moreover, each maternity unit, based on its resources, patient population, and staff skills, devises its own solutions to the same sorts of problems that all other obstetric units share. Unfortunately, there is little collaboration between units to discuss common problems and trade best practices. The result is that all too often each unit invents its own “wheel” when many excellent “wheels” already have been developed for the same issues around the country.
An on-site visit by an outside consultant—an obstetrician, an obstetric nurse, or both—can identify ongoing institutional problems, point out care deficiencies the unit may not be aware of, and provide resources and ideas to help solve the issues identified. Moreover, an outside consultant can offer unbiased and authoritative opinions to help move initiatives that may be stalled by local personalities or institutional politics.
Some features that a well-conducted on-site consultation will evaluate are:
- adequacy of obstetric triage
- capacity to perform stat cesarean sections 24/7
- 24-hour availability of obstetricians, anesthesiologists, pediatricians, and operating room teams
- preparation for handling various obstetric emergencies
- oxytocin administration protocols and compliance
- adequacy of physician and nurse charting
- ongoing skills assessment of fetal heart-rate monitor interpretation
- presence of practitioners whose disruptive behavior compromises the safety of the unit
- preparation for nonmedical emergencies, such as infant abduction, natural disaster; fire; shooter; or disruptive patients, visitors, or staff.
IMPLEMENTATION CAN EQUAL SAFER CARE
As long as people have babies, less than desirable outcomes will occasionally occur. As long as care providers are human beings, the provision of obstetric care will continue to be imperfect.
It is up to those entrusted with the responsibility of caring for mothers and their babies to provide as much support and backup as possible to obstetricians and obstetric nurses, all of whom sincerely desire to do everything possible to deliver safe care to their patients.
Tools for providing such support and backup are available and can be implemented fairly easily on most obstetric units. They do involve an expenditure of both time and money. However, the most important requirement for success is an institutional willingness to 1) acknowledge that the care a given unit provides can be improved, 2) perform an in-depth evaluation of the quality of care currently being administered, and 3) move ahead with the sorts of tools discussed in this article that will enable clinicians to provide optimal care for mothers and babies.
SHARE YOUR EXPERIENCE!
Did implementation of a tool described in this article solve a problem or improve performance for your obstetric unit? Tell us about it by emailing to: obg@frontlinemedcom.com Please include your name, city, and state.
Obstetricians, obstetric nurses, nurse managers, and obstetric department heads are almost always well-trained, hard working, highly motivated individuals dedicated to providing the best possible care for their patients. Nevertheless, errors in the provision of care are all too common.1–3 Even though these errors are confined to a small percentage of patient interactions, they engender profound consequences: injuries to mothers or their babies, higher costs to treat associated complications, and medical-legal suits that can entangle both clinicians and plaintiffs for years.
Why do such errors occur when it is the goal of well-trained and dedicated practi-tioners to provide error-free care? There are several reasons:
- The provision of medical care in the early 21st Century is an enormously complex endeavor.
- Physicians and nurses are human beings and, therefore, do not—and never will—perform perfectly all the time, in every situation, with every patient.
- The systems within which care providers work and the tools with which they work are often suboptimal and inefficient and are not designed to maximize patient safety.
- Financial constraints on hospital systems and physician practices dictate that obstetricians and obstetric nurses care for as many patients as possible in limited periods of time.
How then can obstetrics professionals seek to eradicate or at least decrease the number of medical errors that occur during the provision of maternity care?
To accomplish this, we must address the core issues at the root of these medical errors. Solutions must be implemented to 1) simplify the often unnecessary complexity of delivering medical care and 2) create systems and tools that minimize errors and catch those that do occur before they can cause harm.
Yet, how is this to be accomplished? In this article, I describe eight tools developed over time by clinicians who have worked in the field of obstetric patient safety. These tools provide some answers and concrete starting points.
TOOL 1: CONTINUING EDUCATION
William Osler once said, “It is astonishing with how little reading a doctor can practice medicine, but it is not astonishing how badly he may do it.”
As the years out of residency and nursing school accumulate, clinicians—both obstetricians and obstetric nurses—find it all too easy to continue to practice pretty much the way they did during training. However, medical science changes, new protocols improve on the old, and new techniques and medications are introduced yearly into the practice arena. If a clinician is to deliver the best possible care, he or she has to keep abreast of these developments in obstetrics and refresh his or her memory from time to time about things learned long ago. Such acquisition of new and review of old obstetric knowledge can be achieved only through ongoing study.
There are many ways continuing education can be accomplished. You can read new editions of textbooks when they are published or follow an obstetric journal through its yearly cycle. Cutting-edge, clinically oriented, interactive courses in all major areas of obstetrics are available to clinicians online. The recertification criteria of the American College of Obstetricians and Gynecologists (ACOG), state licensing requirements, and individual obstetric department recredentialing requirements often mandate such continuing education.
TOOL 2: SIMULATION PROGRAMS
Most obstetric emergencies, especially the most dangerous ones, occur infrequently, making it difficult for the many members of any labor and delivery unit to have their skills sharply honed to best deal with them. This is less of a problem at busy institutions where, simply due to the numbers of patients cared for, such emergencies are encountered on a regular basis. But at smaller facilities they are, fortunately, rare. The only way a unit can maintain its competency to handle such situations when they do arise—and they will—is to practice them in simulation mode.
There is now an increasing amount of literature demonstrating that simulation programs are effective not only at improving the knowledge base of obstetrics providers but also at improving Apgar scores, reducing admissions to neonatal intensive care units (NICUs), and preventing brachial plexus injuries.4
An effective simulation program should contain the following features:
- a thorough, didactic review of the clinical aspects of emergency care for all of the major obstetric emergencies (postpartum hemorrhage, shoulder dystocia, eclamptic seizure, maternal collapse, and urgent cesarean section)
- practice drills for the above
- training in teamwork and communication skills
- frequent repetition, ideally with each major obstetric emergency being covered twice per year.
Many institutions have developed simulation training centers. While these can be excellent teaching facilities, something is lost if simulation training is not done on the actual unit where obstetricians and obstetric nurses will encounter emergencies. Simulation programs also should be time-efficient and should be scheduled to make it easy for obstetrics personnel to participate. For greater convenience and knowledge retention, it is better to have short simulation programs at frequent intervals than day-long programs once per year or every other year.
Related Article: How simulation can train, and refresh, physicians for critical OB events Robert Gherman, MD; Andrew Satin, MD; Roxane Gardner, MD, MPH
TOOL 3: INTERNAL AUDITS
It is a mantra in business that you can’t fix what you can’t measure. And while obstetric units usually keep track of such things as rates of cesarean section, elective induction at less than 39 weeks, and admission to the NICU, it is rare that data are kept on other extremely important information. For instance, how often is an induction started with no indication for it written in the admission note? How often is the vacuum or forceps applied with no note documenting the reason or the discussion of risks and benefits with the patient? How often does estimated fetal weight go unnoted in the medical record of a mother with gestational diabetes?
An audit program, either in computer format or with manual collection on paper, is a vital tool for each labor and delivery unit to use in assessing the quality of the care it provides. Such an audit, by covering a sufficiently large number of clinical data points, can give tremendous insight into the specifics of the unit’s performance over the range of obstetric care situations. It will show where things are being done well and where they are not. The audit becomes even more valuable if it is designed so that each of the measured data points can be evaluated for individual clinician performance as well as for the labor and delivery unit as a whole.
Similar audits also should be conducted in individual physician offices and obstetric clinics. Many of the errors that occur in providing obstetric care occur prenatally: tests not performed, lack of follow-up of known problems, or poor communication with patients or with the labor and delivery unit.
One of the major benefits of audit programs that are conducted on a regular basis—every 6 months or annually are common intervals—is that trends in performance in each area of care can be evaluated. As deficiencies are pointed out to providers, their compliance with best care practices should improve from cycle to cycle.
TOOL 4: BEST PRACTICE PROTOCOLS
Medicine is now well past the point where protocols are seen as “restrictive” or “advocating cookbook medicine.” Well-designed protocols summarize best practices derived from evidence-based studies and the consensus of obstetric experts. They serve as convenient reminders to physicians in various clinical situations so that these clinicians do not have to rely solely on what they happen to remember about caring for a given condition. Protocols also provide a certain uniformity of care, which in itself decreases the likelihood of errors being made.
Each obstetric department should have a set of protocols to cover the most common obstetric situations, such as:
- premature rupture of membranes
- instrumental vaginal deliveries
- oxytocin administration.
Each unit does not have to devise its own protocols; ACOG and nearby academic institutions are excellent sources for protocols that can be replicated and implemented so that they do not have to be created de novo.
Related Article: More strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD; Alexis C. Gimovsky, MD (January 2011)
TOOL 5: SAFETY CHECKLISTS
Just as well-designed protocols can serve as convenient reminders of best practices, low-tech physical checklists can be kept at nursing stations and in labor and delivery rooms to serve as reminders of best practices during obstetric emergencies. For instance, having a laminated set of easy-to-read protocols for postpartum hemorrhage, eclamptic seizure, maternal collapse, and shoulder dystocia in a delivery room can allow a charge nurse or other supervisor to check to make sure all proper procedures are being performed by the team actually administering care to a patient in crisis, with nothing important overlooked.
Related Article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations Robert L. Barbieri, MD (Editorial, August 2013)
TOOL 6: COMPLETE DOCUMENTATION
Almost as many lawsuits are lost because of poor documentation as are lost because of inappropriate medical care. The obstetric literature,1 and my own experience with the medical-legal system, clearly demonstrate the need for appropriate, careful documentation of the events that transpire during patient care. Notes do not have to be especially long or verbose—but they must contain all relevant information and describe the obstetrician’s thinking at various decision points.
Documentation can be inadequate because of time constraints, poor understanding of the events that transpired, or simply a lack of remembering to include salient points that should be covered in a clinical note.
Clinicians can be prompted to include key aspects of care in the medical record by using prepared templates. Such templates are easy to fill out, remind clinicians to document information that would otherwise not get recorded, and result in a much more complete patient chart. By using a template, a clinician would never forget to record the head-to-body delivery interval after a shoulder dystocia or whether a fetal heart rate was obtained in the operating room just prior to starting a cesarean section.
Related Article: Sound strategies to avoid malpractice hazards on labor and delivery Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD (December 2010)
TOOL 7: SMART MEDICAL RECORDS
In obstetrics we are fortunate that there is a limited range of issues that recur repeatedly, such as gestational hypertension, placental abruption, and fetal distress. One soon gains experience in managing these conditions and, with the help of best-practice protocols, optimal care almost always can be provided.
Still, many clinical presentations can pose diagnostic challenges, especially in atypical cases. Moreover, clinicians managing a patient’s care may not immediately remember the best means of evaluating and treating a certain condition in specific circumstances. For example, at 3:00 am it may be difficult to recall whether it is nifedipine or labetalol that should be avoided with asthmatic patients or which antibiotic formulation is currently recommended for prophylaxis in a patient with premature rupture of membranes at 30 weeks’ gestation who is allergic to penicillin.
Smart medical records, already widely used in other fields of medicine, are an antidote to this problem. When certain diagnoses, physical findings, clinical details, or laboratory data are entered into specific fields in an electronic medical record, templates that have been added to the record automatically appear to show relevant information, such as tests that should be performed, treatments that should be administered, and alternative diagnoses that should be considered. Such reminders are not presented as obligations or “hard stops”; they are usually displayed in the form of easily dismissible pop-ups or “reminder bubbles” that appear on the screen and serve solely to jog memory and provide information.
Such smart electronic medical record features can be provided either by the main electronic medical record vendor or added as subprograms by other providers.
Related Article: EHRs and medicolegal risk: How they help, when they could hurt Martin L. Gimovsky, MD; Baohuong N. Trans, DO (March 2013)
TOOL 8: MATERNITY UNIT ON-SITE CONSULTATIONS
Every labor and delivery unit has its own culture, a combination of institutional history and the personality of the doctors and nurses working there. Some units function efficiently, have the most modern equipment, and provide superb medical care. Other units have less than adequate facilities, remain entrenched in older practices, and have disruptive or uncooperative personnel that interfere with the smooth running of the unit. Moreover, each maternity unit, based on its resources, patient population, and staff skills, devises its own solutions to the same sorts of problems that all other obstetric units share. Unfortunately, there is little collaboration between units to discuss common problems and trade best practices. The result is that all too often each unit invents its own “wheel” when many excellent “wheels” already have been developed for the same issues around the country.
An on-site visit by an outside consultant—an obstetrician, an obstetric nurse, or both—can identify ongoing institutional problems, point out care deficiencies the unit may not be aware of, and provide resources and ideas to help solve the issues identified. Moreover, an outside consultant can offer unbiased and authoritative opinions to help move initiatives that may be stalled by local personalities or institutional politics.
Some features that a well-conducted on-site consultation will evaluate are:
- adequacy of obstetric triage
- capacity to perform stat cesarean sections 24/7
- 24-hour availability of obstetricians, anesthesiologists, pediatricians, and operating room teams
- preparation for handling various obstetric emergencies
- oxytocin administration protocols and compliance
- adequacy of physician and nurse charting
- ongoing skills assessment of fetal heart-rate monitor interpretation
- presence of practitioners whose disruptive behavior compromises the safety of the unit
- preparation for nonmedical emergencies, such as infant abduction, natural disaster; fire; shooter; or disruptive patients, visitors, or staff.
IMPLEMENTATION CAN EQUAL SAFER CARE
As long as people have babies, less than desirable outcomes will occasionally occur. As long as care providers are human beings, the provision of obstetric care will continue to be imperfect.
It is up to those entrusted with the responsibility of caring for mothers and their babies to provide as much support and backup as possible to obstetricians and obstetric nurses, all of whom sincerely desire to do everything possible to deliver safe care to their patients.
Tools for providing such support and backup are available and can be implemented fairly easily on most obstetric units. They do involve an expenditure of both time and money. However, the most important requirement for success is an institutional willingness to 1) acknowledge that the care a given unit provides can be improved, 2) perform an in-depth evaluation of the quality of care currently being administered, and 3) move ahead with the sorts of tools discussed in this article that will enable clinicians to provide optimal care for mothers and babies.
SHARE YOUR EXPERIENCE!
Did implementation of a tool described in this article solve a problem or improve performance for your obstetric unit? Tell us about it by emailing to: obg@frontlinemedcom.com Please include your name, city, and state.
- Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279–1283.
- Gluck PA. Medical error theory. Obstet Gynecol Clin North Am. 2008;35(1):11–17, vii.
- Anakiraman V, Ecker J. Quality in obstetric care: measuring what matters. Obstet Gynecol. 2010;116(3):728–732.
- Draycott T, Sibanda T, Owen L, et al. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113(2):177–182.
- Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279–1283.
- Gluck PA. Medical error theory. Obstet Gynecol Clin North Am. 2008;35(1):11–17, vii.
- Anakiraman V, Ecker J. Quality in obstetric care: measuring what matters. Obstet Gynecol. 2010;116(3):728–732.
- Draycott T, Sibanda T, Owen L, et al. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113(2):177–182.
Rebuff those malpractice lawyers’ traps and tricks!
The author reports that he is president of Shoulder Dystocia Litigation Consultants, working with defense lawyers, insurance company case managers, and hospital risk managers in shoulder dystocia-related injuries and litigation.
CASE
You are a defendant in a malpractice case, and your lawyer has just finished questioning you—the “direct” part of your testimony. She asked you straightforward questions and you answered fully and without interruption. You were able to explain, at length, your account of what happened during the events in question. This is the first time you’ve been sued; you’re nervous, but things have gone well so far, you feel.
Cross-examination by the plaintiff’s attorney comes next. He starts aggressively, questioning the quality of your training and experience. Have any disciplinary actions ever been taken against you by your hospital or the state licensing board? Did you have specialty fellowship training? He makes it seem that, if you didn’t, you have no business taking care of patients.
He drills in: Have you taken courses in the specific area at question in the case—as if whole courses are given routinely on the narrow topics that are often the subject of litigation, whether shoulder dystocia, placental abruption, damage to a ureter, or other bad outcomes.
He moves on to ask about details of the case but cuts you off when you try to flesh out your answers. He admonishes you: Listen to the question and answer “Yes” or “No”!
He begins to raise his voice.
The attorney attacks your notes in the medical record; he makes them seem incomplete and inadequate. He tells members of the jury that they can assume that you did not take a specific action, despite your claim to the contrary, because it’s not in the record: “If it wasn’t written down, it didn’t happen.”
His demeanor becomes more confrontational. The increasingly abusive questioning goes on and on, and your sense that things are going well has evaporated.
How, you ask yourself as the assault continues, did all this rancor and accusation come on so fast and so unexpectedly?
This scenario, or versions close to it, occurs all too often to physicians in courtrooms across the United States. Defendant physicians who are vilified and goaded feel angry, frustrated, and helpless. No wonder—the courtroom environment is alien to us. We trained for years to become competent, knowledgeable practitioners of our specialty; we work hard every day to provide the best possible care; and we diligently keep up with advances in ObGyn medicine by reading the literature and attending continuing medical education conferences. But in the courtroom, attorneys make a pointed attempt to paint us as incompetent and uncaring—even malicious.
Moreover, customary rules of argumentation don’t apply. We can’t answer questions fully or correct misstatements that are implicit in certain questions. Judges often limit what we can say and what the jury is allowed to hear. Not only is the medical care we gave questioned—we are subject to attempts to discredit us personally. We’re asked questions about the most private aspects of our life: “What’s your income?” “Why were you divorced?” “What is the financial arrangement between you and your partners?” “Are you seeing—have you ever seen—a psychiatrist?”
The playing field has been set at a tilt
Perhaps your greatest disadvantage when you are sued is that, most likely, this is going to be your first time in a courtroom. You haven’t had the chance to become familiar with the venue—the courtroom—or the tactics of cross-examination used by plaintiff attorneys.
Combine an accusation of malpractice and the need to defend yourself in an alien environment with rules made by and favoring lawyers that are foreign to you and that you cannot control—what a daunting prospect! Plaintiff attorneys take advantage of the situation to prey on defendants.
There are ways to defend yourself!
Did you go into an operating room or a delivery room for the first time without preparation or training? No! Likewise, don’t go into a courtroom unprepared.
You may be surprised to learn that you do have advantages over lawyers for plaintiffs:
- You know more medicine than they ever will, no matter how many malpractice cases they have tried.
- You were there when the actions under dispute took place. You can speak from direct experience about those actions, with authority, as a knowledgeable eyewitness.
- Despite how it may appear, you have the right to defend your actions and your statements vigorously.
Plaintiff lawyers routinely employ a standard repertoire of tricks and traps, which I have seen used time and again. My goals here are to describe them to you so that you can see them coming and to tell you how to defend yourself against them. You’ll then be in a position to counter these tricks by 1) giving them a name, 2) confronting the lawyer—in front of the judge and the jury—with what he or she is attempting to do, and 3) employing defensive tactics.
A note about language in this article: For simplicity, when I say “he” when referring to a physician or lawyer, I mean “he” or “she.” And I mean “plaintiff attorney” when I say just “lawyer” or “attorney,” unless I am referring explicitly to your (the defendant’s) representation.
First, three little words to set the stage
Always keep in mind that, for you to be found guilty of malpractice, the plaintiff attorney has to prove beyond a reasonable doubt that the actions you did, or did not, take violated what is known in the medicolegal arena as standard of care. Because this standard is what you are being judged against, it is vital that you understand—and, in turn, that the jury understands—exactly what the term means.
Standard of care is defined as care generally given by well-trained physicians in your own specialty under similar circumstances. Standard of care does not mean “ideal” care, as may be recommended in a medical textbook or other kinds of professional communication. The standard of care is, essentially, generally accepted practice: The level and degree of care most often used by your contemporary peers. You are guilty of malpractice only if the care that you gave fell below the care that would generally have been given to a patient by others, in your specialty, under the circumstances you faced.
Inside an attorney’s bulging bag of tricks
What tactics might an attorney use to harass and intimidate you?
He’ll bully you. Imagine this: A plaintiff lawyer is brought into a surgical suite for the first time. He is asked to participate in an operation but isn’t allowed to speak unless spoken to. He is allowed to answer direct questions only in a format dictated by the senior surgeon. That lawyer would not know what was going on, would be continuously on the defensive, and would feel totally in over his head—if he didn’t faint first!
What I just described is the equivalent of what happens to you in a courtroom. An attorney is allowed great leeway over the types of questions that he can ask and the manner in which they can be posed. He often attempts to intimidate you with harsh language, a raised voice, physical gestures, and sarcasm. He might ask questions with implied premises that aren’t true. His behavior might be confrontational. He might try to cut you off. And he might insist that your answers be solely “Yes” or “No.”
He’ll troll through your CV. Every educational activity in which you have participated, and every professional position you have ever held, is subject to inquiry. In addition to being asked if you have ever been sued or had disciplinary action taken against you, an attorney will review your education, step by step. He might imply that, if you were educated abroad or went to a less-than-well-known medical school, you are poorly trained or somehow not “of high quality.” He will likely ask you how many times you took the specialty board exam before you passed it. You might even be asked how high you finished in your medical school class, or if you were given your first choice of residency program in a match.
He’ll create artificial standards in the minds of jury members. You might be asked if you have published in your field or if you have an academic appointment—the assumption being that, if your answer is “No,” your opinion about issues being discussed at the trial are not as authoritative as (he will claim) those of the plaintiff’s expert witness, who may be well known in the specialty.
He’ll take statements out of context. Articles that you published (even if years ago), previous depositions or trial testimony you have given, and even PowerPoint presentations you made to nurses on your labor and delivery unit may be probed and quoted. Usually, the attorney presents only brief snippets of these works, which are likely to be read to the jury out of context.
He’ll ask for specific references. Often, when an attorney asks about facts that you’ve mentioned or opinions you hold regarding issues that bear on the case in your trial, he will attempt to embarrass you by asking you to name the specific text, article, or author from which you obtained that information. Here’s an example: You know that the threshold for macrosomia in a shoulder dystocia case is 4,500 g, and that random late decelerations in a fetal monitor strip marked by otherwise excellent variability do not demand immediate C-section—but you may not be able to cite, off the top of your head, exactly in which textbook or journal article you read this or the information can be found. You might also be asked what an ACOG Bulletin or your hospital’s policy book says about a certain subject or aspect of care.
He’ll drag in the medical record and informed consent. An attorney might try to convince a jury that “if it isn’t written down, it didn’t happen.” He might cite a lack of an extensive written description of what occurred during the events in question as evidence of sloppy charting or poor care. He might claim that lack of a detailed note replicating a conversation that took place during the consent process displays a lack of concern for the patient’s right to know.
He’ll imply the existence of a standard of care. Lawyers often try to convince a jury that a defendant physician committed malpractice by claiming that she should have taken certain actions, when, in fact, these actions would have been unnecessary or inappropriate under the circumstances. Examples: Asking whether clinical pelvimetry was documented in the chart of a multiparous woman who came in actively laboring, or asking if fundal height was measured in the office during a patient’s last three prenatal visits.
Here are two other examples:
- In a case involving vacuum extraction delivery: “Doctor, have you ever read the vacuum device’s product safety manual?”
- When a plaintiff has testified that she told you at her first prenatal visit that her previous pregnancies were uncomplicated: “Did you call for, or read, the record from any of her previous pregnancies?”
He’ll create a false impression. A common attorney’s tactic is to pose questions to you that imply that certain things are true, when they are not. A common example of this tactic occurs in shoulder dystocia cases, when putative risk factors are addressed.
Consensus in the shoulder dystocia literature is that there are only three or four statistically consistent risk factors for this condition: shoulder dystocia in a prior delivery, macrosomia, gestational diabetes, and (possibly) mid-vacuum or forceps delivery. Often, however, attorneys imply to the jury that many other risk factors exist—and that your patient had any number of them and that you should have been aware of them.
You might be asked if your patient underwent oxytocin induction, had a long first stage of labor, had an epidural anesthetic placed, or was post-dates—none of which have a proven association with shoulder dystocia. You’ll be given little leeway, in answering questions posed to you, to try to refute the lawyer’s false assumptions. The impression may thus be left by this concatenation of nonproven factors that your patient was at high risk of shoulder dystocia, that this was foreseeable, and that you were negligent in not having performed a C-section to prevent it.
Likewise, lawyers often deliberately misuse statistics—such as when they discuss sonographic variability in the estimation of fetal weight: “Don’t you acknowledge, Doctor, that ultrasound estimates of fetal weight can vary by 15% of the actual weight? So why didn’t you take into account that the 4,300 g estimate you were given could, in fact, have been as high as 4,700 g?” Given the rules that restrict how you can answer, you are rarely allowed to explain to a jury that, first, the 15% variability applies only to a baby whose weight is more than one standard deviation from average and, second, the weight-estimate variability can be on the low side as well as on the high side.
How should you respond to interrogation?
Although you face disadvantages as a defendant physician in a courtroom, there are ways to fight back—to stick up for yourself and respond to the techniques that attorneys perpetrate. You aren’t as defenseless as it might appear!
Never allow an attorney to bully you in the courtroom or at a deposition. If the attorney begins to use such behavior, call it by its name and demand that it be stopped. Your lawyer will likely have raised an objection before you do; if she does not, protest such inappropriate behavior yourself. Never allow an attorney who is questioning you to raise his voice or speak to you sarcastically or rudely.
You don’t necessarily have to play by the rules for answering questions, despite any admonition by a plaintiff lawyer that you do so. Unless you are advised otherwise by the judge or by your lawyer, answer questions the way you want to, as long as your answer is a reply to the question that was asked. You are never obliged to answer a question with just “Yes” or “No.” If an attorney tries to impose such a limitation on you, declare that you cannot answer the question under those terms. If your answers are being cut off, don’t hesitate to tell the jury that you are not being allowed to tell the whole story.
If questions posed to you contain false premises, point that out. For example, you might be asked, “Given the obvious fetal distress that was present, why did you apply forceps?” If there was no fetal distress, or if that is one of the issues in dispute, you can respond that the question contains incorrect information or an unwarranted assumption, and therefore cannot be answered as asked.
Prepare to be asked about your background and training. Have your lawyer ask you preemptively, during her questioning, about anything in your professional life that might appear the least bit negative. This allows you to explain the matter fully without being cut off by the plaintiff attorney. Have your lawyer ask questions that show how your background and training compare with those of other physicians in your hospital and community. If you have been sued in the past, have your lawyer ask you about how many times an ObGyn is sued, on average, in her career (“three” is the answer), and use this fact to show the jury that being sued is not an anomaly but the rule in ObGyn practice.
Never answer a question about something you wrote in the past or about prior testimony without demanding to read it yourself, on the stand, in context. (The same is true for quotations from the medical literature read to you by the plaintiff attorney: You have a right to know the source and date of publication of quoted material, and you should insist on being able to read the quotation for yourself so that you can understand it in context.)
If asked from what text or article you learned a specific piece of information, point out the absurdity of being asked to remember such specifics from among the tens of thousands of things you have learned and read over your training and career.
When asked about your notes in the medical record and why you did or did not write a particular item, point out that the medical record is not a document that is intended to be used to prosecute or defend medical cases years down the road but rather is meant to convey important clinical information among health-care providers. Tell the jury what sorts of notes are routinely written and how much information is generally put into a note. If the notes you wrote are appropriate, even if brief, be sure and explain to the jury that what you did is, in fact, the standard of care—not an idealized conception taken from a textbook or an expert’s talk as to how notes should be written. Don’t agree with a lawyer’s contention that “if it isn’t written down, it didn’t happen.” That may be a lawyer’s rule; it is not a medical rule. Do not let the jury go into the jury room thinking that it is.
Know the specifics of your case. It is true that, as a defendant witness, several factors are out of your control. But don’t forget what you do have under your control: Knowledge of obstetrics and gynecology and experience in the field. You know the medical issues involved in the litigation better than anyone else in the courtroom.
Still, do your homework. Make sure that you know the specifics of your case, inside and out. Study the medical record of the case carefully and read all the depositions your lawyer provides for you. Know what the relevant ACOG Bulletin, major texts (such as Williams Obstetrics), and the literature say about the issues that are involved. Know who the experts are in this area of care and be prepared to quote pertinent articles that they have written. Work to never let yourself be surprised by the facts of the case or the medical information presented by the plaintiff’s side. Treat your testimony as a very important final examination. Do that, and you will be in an excellent position to successfully answer questions and refute incorrect statements.
Preempt questions about informed consent. Ask your lawyer to have you explain, during the direct portion of your testimony, about informed consent conversations, how they are usually held, and how they are documented. Tell the jury the difference between a calm consent discussion in the office before a routine medical procedure and a consent discussion in an urgent situation. By the way: The general rule about informed consent is that a physician is obliged to discuss with a patient any significant risk greater than 1%. This is a documented standard.1
Don’t let incorrect claims go unchallenged. Consider this scenario: A plaintiff attorney states that, given the circumstances of a certain clinical situation, you should have taken a particular action. This is often the case in fetal asphyxia cases, when experts for the plaintiff often testify that they can tell, from looking at the fetal heart rate monitoring strip, the exact moment at which a fetus was in trouble and should have been delivered by C-section. Consider having your lawyer issue an in-court challenge to an expert witness who makes such a claim to perform a blind reading of five fetal monitor strips for which the outcomes are known and to see if his predictions are correct. A plaintiff attorney will never take you up on such a challenge—and that refusal will be noted and appreciated by the jury.
This isn’t your backyard but you can play here
Amid what is often hostile treatment, it can be difficult to remember who you are: A highly trained, hard-working physician who has given most of your professional life to providing superb care. A plaintiff attorney is out to make you appear incompetent, and his motive is clear: He’ll earn one-quarter to one-third of any award that he wins for his client.
You are obviously convinced of the correctness of what you did in the case—or you wouldn’t have gone to court to defend yourself. You know the medicine better than the plaintiff lawyer does and, having been the caregiver, you can discuss all aspects of the case with much greater authority than he ever can. His only advantage? You’re in his backyard and he controls many of the rules.
But if you’re meticulously prepared, if you work with your lawyer and follow her advice, and if you are aware of the plaintiff attorneys’ tricks and techniques that I’ve described, you can neutralize much of the disadvantage you’re under in the legal system and defend your case on a greatly leveled playing field.
Reference
1. Nichols DL, Caldwell JW. Medicolegal complications consequent to unauthorized surgery. In: Nichols DH, DeLancey JOL, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1995:445-447.
The author reports that he is president of Shoulder Dystocia Litigation Consultants, working with defense lawyers, insurance company case managers, and hospital risk managers in shoulder dystocia-related injuries and litigation.
CASE
You are a defendant in a malpractice case, and your lawyer has just finished questioning you—the “direct” part of your testimony. She asked you straightforward questions and you answered fully and without interruption. You were able to explain, at length, your account of what happened during the events in question. This is the first time you’ve been sued; you’re nervous, but things have gone well so far, you feel.
Cross-examination by the plaintiff’s attorney comes next. He starts aggressively, questioning the quality of your training and experience. Have any disciplinary actions ever been taken against you by your hospital or the state licensing board? Did you have specialty fellowship training? He makes it seem that, if you didn’t, you have no business taking care of patients.
He drills in: Have you taken courses in the specific area at question in the case—as if whole courses are given routinely on the narrow topics that are often the subject of litigation, whether shoulder dystocia, placental abruption, damage to a ureter, or other bad outcomes.
He moves on to ask about details of the case but cuts you off when you try to flesh out your answers. He admonishes you: Listen to the question and answer “Yes” or “No”!
He begins to raise his voice.
The attorney attacks your notes in the medical record; he makes them seem incomplete and inadequate. He tells members of the jury that they can assume that you did not take a specific action, despite your claim to the contrary, because it’s not in the record: “If it wasn’t written down, it didn’t happen.”
His demeanor becomes more confrontational. The increasingly abusive questioning goes on and on, and your sense that things are going well has evaporated.
How, you ask yourself as the assault continues, did all this rancor and accusation come on so fast and so unexpectedly?
This scenario, or versions close to it, occurs all too often to physicians in courtrooms across the United States. Defendant physicians who are vilified and goaded feel angry, frustrated, and helpless. No wonder—the courtroom environment is alien to us. We trained for years to become competent, knowledgeable practitioners of our specialty; we work hard every day to provide the best possible care; and we diligently keep up with advances in ObGyn medicine by reading the literature and attending continuing medical education conferences. But in the courtroom, attorneys make a pointed attempt to paint us as incompetent and uncaring—even malicious.
Moreover, customary rules of argumentation don’t apply. We can’t answer questions fully or correct misstatements that are implicit in certain questions. Judges often limit what we can say and what the jury is allowed to hear. Not only is the medical care we gave questioned—we are subject to attempts to discredit us personally. We’re asked questions about the most private aspects of our life: “What’s your income?” “Why were you divorced?” “What is the financial arrangement between you and your partners?” “Are you seeing—have you ever seen—a psychiatrist?”
The playing field has been set at a tilt
Perhaps your greatest disadvantage when you are sued is that, most likely, this is going to be your first time in a courtroom. You haven’t had the chance to become familiar with the venue—the courtroom—or the tactics of cross-examination used by plaintiff attorneys.
Combine an accusation of malpractice and the need to defend yourself in an alien environment with rules made by and favoring lawyers that are foreign to you and that you cannot control—what a daunting prospect! Plaintiff attorneys take advantage of the situation to prey on defendants.
There are ways to defend yourself!
Did you go into an operating room or a delivery room for the first time without preparation or training? No! Likewise, don’t go into a courtroom unprepared.
You may be surprised to learn that you do have advantages over lawyers for plaintiffs:
- You know more medicine than they ever will, no matter how many malpractice cases they have tried.
- You were there when the actions under dispute took place. You can speak from direct experience about those actions, with authority, as a knowledgeable eyewitness.
- Despite how it may appear, you have the right to defend your actions and your statements vigorously.
Plaintiff lawyers routinely employ a standard repertoire of tricks and traps, which I have seen used time and again. My goals here are to describe them to you so that you can see them coming and to tell you how to defend yourself against them. You’ll then be in a position to counter these tricks by 1) giving them a name, 2) confronting the lawyer—in front of the judge and the jury—with what he or she is attempting to do, and 3) employing defensive tactics.
A note about language in this article: For simplicity, when I say “he” when referring to a physician or lawyer, I mean “he” or “she.” And I mean “plaintiff attorney” when I say just “lawyer” or “attorney,” unless I am referring explicitly to your (the defendant’s) representation.
First, three little words to set the stage
Always keep in mind that, for you to be found guilty of malpractice, the plaintiff attorney has to prove beyond a reasonable doubt that the actions you did, or did not, take violated what is known in the medicolegal arena as standard of care. Because this standard is what you are being judged against, it is vital that you understand—and, in turn, that the jury understands—exactly what the term means.
Standard of care is defined as care generally given by well-trained physicians in your own specialty under similar circumstances. Standard of care does not mean “ideal” care, as may be recommended in a medical textbook or other kinds of professional communication. The standard of care is, essentially, generally accepted practice: The level and degree of care most often used by your contemporary peers. You are guilty of malpractice only if the care that you gave fell below the care that would generally have been given to a patient by others, in your specialty, under the circumstances you faced.
Inside an attorney’s bulging bag of tricks
What tactics might an attorney use to harass and intimidate you?
He’ll bully you. Imagine this: A plaintiff lawyer is brought into a surgical suite for the first time. He is asked to participate in an operation but isn’t allowed to speak unless spoken to. He is allowed to answer direct questions only in a format dictated by the senior surgeon. That lawyer would not know what was going on, would be continuously on the defensive, and would feel totally in over his head—if he didn’t faint first!
What I just described is the equivalent of what happens to you in a courtroom. An attorney is allowed great leeway over the types of questions that he can ask and the manner in which they can be posed. He often attempts to intimidate you with harsh language, a raised voice, physical gestures, and sarcasm. He might ask questions with implied premises that aren’t true. His behavior might be confrontational. He might try to cut you off. And he might insist that your answers be solely “Yes” or “No.”
He’ll troll through your CV. Every educational activity in which you have participated, and every professional position you have ever held, is subject to inquiry. In addition to being asked if you have ever been sued or had disciplinary action taken against you, an attorney will review your education, step by step. He might imply that, if you were educated abroad or went to a less-than-well-known medical school, you are poorly trained or somehow not “of high quality.” He will likely ask you how many times you took the specialty board exam before you passed it. You might even be asked how high you finished in your medical school class, or if you were given your first choice of residency program in a match.
He’ll create artificial standards in the minds of jury members. You might be asked if you have published in your field or if you have an academic appointment—the assumption being that, if your answer is “No,” your opinion about issues being discussed at the trial are not as authoritative as (he will claim) those of the plaintiff’s expert witness, who may be well known in the specialty.
He’ll take statements out of context. Articles that you published (even if years ago), previous depositions or trial testimony you have given, and even PowerPoint presentations you made to nurses on your labor and delivery unit may be probed and quoted. Usually, the attorney presents only brief snippets of these works, which are likely to be read to the jury out of context.
He’ll ask for specific references. Often, when an attorney asks about facts that you’ve mentioned or opinions you hold regarding issues that bear on the case in your trial, he will attempt to embarrass you by asking you to name the specific text, article, or author from which you obtained that information. Here’s an example: You know that the threshold for macrosomia in a shoulder dystocia case is 4,500 g, and that random late decelerations in a fetal monitor strip marked by otherwise excellent variability do not demand immediate C-section—but you may not be able to cite, off the top of your head, exactly in which textbook or journal article you read this or the information can be found. You might also be asked what an ACOG Bulletin or your hospital’s policy book says about a certain subject or aspect of care.
He’ll drag in the medical record and informed consent. An attorney might try to convince a jury that “if it isn’t written down, it didn’t happen.” He might cite a lack of an extensive written description of what occurred during the events in question as evidence of sloppy charting or poor care. He might claim that lack of a detailed note replicating a conversation that took place during the consent process displays a lack of concern for the patient’s right to know.
He’ll imply the existence of a standard of care. Lawyers often try to convince a jury that a defendant physician committed malpractice by claiming that she should have taken certain actions, when, in fact, these actions would have been unnecessary or inappropriate under the circumstances. Examples: Asking whether clinical pelvimetry was documented in the chart of a multiparous woman who came in actively laboring, or asking if fundal height was measured in the office during a patient’s last three prenatal visits.
Here are two other examples:
- In a case involving vacuum extraction delivery: “Doctor, have you ever read the vacuum device’s product safety manual?”
- When a plaintiff has testified that she told you at her first prenatal visit that her previous pregnancies were uncomplicated: “Did you call for, or read, the record from any of her previous pregnancies?”
He’ll create a false impression. A common attorney’s tactic is to pose questions to you that imply that certain things are true, when they are not. A common example of this tactic occurs in shoulder dystocia cases, when putative risk factors are addressed.
Consensus in the shoulder dystocia literature is that there are only three or four statistically consistent risk factors for this condition: shoulder dystocia in a prior delivery, macrosomia, gestational diabetes, and (possibly) mid-vacuum or forceps delivery. Often, however, attorneys imply to the jury that many other risk factors exist—and that your patient had any number of them and that you should have been aware of them.
You might be asked if your patient underwent oxytocin induction, had a long first stage of labor, had an epidural anesthetic placed, or was post-dates—none of which have a proven association with shoulder dystocia. You’ll be given little leeway, in answering questions posed to you, to try to refute the lawyer’s false assumptions. The impression may thus be left by this concatenation of nonproven factors that your patient was at high risk of shoulder dystocia, that this was foreseeable, and that you were negligent in not having performed a C-section to prevent it.
Likewise, lawyers often deliberately misuse statistics—such as when they discuss sonographic variability in the estimation of fetal weight: “Don’t you acknowledge, Doctor, that ultrasound estimates of fetal weight can vary by 15% of the actual weight? So why didn’t you take into account that the 4,300 g estimate you were given could, in fact, have been as high as 4,700 g?” Given the rules that restrict how you can answer, you are rarely allowed to explain to a jury that, first, the 15% variability applies only to a baby whose weight is more than one standard deviation from average and, second, the weight-estimate variability can be on the low side as well as on the high side.
How should you respond to interrogation?
Although you face disadvantages as a defendant physician in a courtroom, there are ways to fight back—to stick up for yourself and respond to the techniques that attorneys perpetrate. You aren’t as defenseless as it might appear!
Never allow an attorney to bully you in the courtroom or at a deposition. If the attorney begins to use such behavior, call it by its name and demand that it be stopped. Your lawyer will likely have raised an objection before you do; if she does not, protest such inappropriate behavior yourself. Never allow an attorney who is questioning you to raise his voice or speak to you sarcastically or rudely.
You don’t necessarily have to play by the rules for answering questions, despite any admonition by a plaintiff lawyer that you do so. Unless you are advised otherwise by the judge or by your lawyer, answer questions the way you want to, as long as your answer is a reply to the question that was asked. You are never obliged to answer a question with just “Yes” or “No.” If an attorney tries to impose such a limitation on you, declare that you cannot answer the question under those terms. If your answers are being cut off, don’t hesitate to tell the jury that you are not being allowed to tell the whole story.
If questions posed to you contain false premises, point that out. For example, you might be asked, “Given the obvious fetal distress that was present, why did you apply forceps?” If there was no fetal distress, or if that is one of the issues in dispute, you can respond that the question contains incorrect information or an unwarranted assumption, and therefore cannot be answered as asked.
Prepare to be asked about your background and training. Have your lawyer ask you preemptively, during her questioning, about anything in your professional life that might appear the least bit negative. This allows you to explain the matter fully without being cut off by the plaintiff attorney. Have your lawyer ask questions that show how your background and training compare with those of other physicians in your hospital and community. If you have been sued in the past, have your lawyer ask you about how many times an ObGyn is sued, on average, in her career (“three” is the answer), and use this fact to show the jury that being sued is not an anomaly but the rule in ObGyn practice.
Never answer a question about something you wrote in the past or about prior testimony without demanding to read it yourself, on the stand, in context. (The same is true for quotations from the medical literature read to you by the plaintiff attorney: You have a right to know the source and date of publication of quoted material, and you should insist on being able to read the quotation for yourself so that you can understand it in context.)
If asked from what text or article you learned a specific piece of information, point out the absurdity of being asked to remember such specifics from among the tens of thousands of things you have learned and read over your training and career.
When asked about your notes in the medical record and why you did or did not write a particular item, point out that the medical record is not a document that is intended to be used to prosecute or defend medical cases years down the road but rather is meant to convey important clinical information among health-care providers. Tell the jury what sorts of notes are routinely written and how much information is generally put into a note. If the notes you wrote are appropriate, even if brief, be sure and explain to the jury that what you did is, in fact, the standard of care—not an idealized conception taken from a textbook or an expert’s talk as to how notes should be written. Don’t agree with a lawyer’s contention that “if it isn’t written down, it didn’t happen.” That may be a lawyer’s rule; it is not a medical rule. Do not let the jury go into the jury room thinking that it is.
Know the specifics of your case. It is true that, as a defendant witness, several factors are out of your control. But don’t forget what you do have under your control: Knowledge of obstetrics and gynecology and experience in the field. You know the medical issues involved in the litigation better than anyone else in the courtroom.
Still, do your homework. Make sure that you know the specifics of your case, inside and out. Study the medical record of the case carefully and read all the depositions your lawyer provides for you. Know what the relevant ACOG Bulletin, major texts (such as Williams Obstetrics), and the literature say about the issues that are involved. Know who the experts are in this area of care and be prepared to quote pertinent articles that they have written. Work to never let yourself be surprised by the facts of the case or the medical information presented by the plaintiff’s side. Treat your testimony as a very important final examination. Do that, and you will be in an excellent position to successfully answer questions and refute incorrect statements.
Preempt questions about informed consent. Ask your lawyer to have you explain, during the direct portion of your testimony, about informed consent conversations, how they are usually held, and how they are documented. Tell the jury the difference between a calm consent discussion in the office before a routine medical procedure and a consent discussion in an urgent situation. By the way: The general rule about informed consent is that a physician is obliged to discuss with a patient any significant risk greater than 1%. This is a documented standard.1
Don’t let incorrect claims go unchallenged. Consider this scenario: A plaintiff attorney states that, given the circumstances of a certain clinical situation, you should have taken a particular action. This is often the case in fetal asphyxia cases, when experts for the plaintiff often testify that they can tell, from looking at the fetal heart rate monitoring strip, the exact moment at which a fetus was in trouble and should have been delivered by C-section. Consider having your lawyer issue an in-court challenge to an expert witness who makes such a claim to perform a blind reading of five fetal monitor strips for which the outcomes are known and to see if his predictions are correct. A plaintiff attorney will never take you up on such a challenge—and that refusal will be noted and appreciated by the jury.
This isn’t your backyard but you can play here
Amid what is often hostile treatment, it can be difficult to remember who you are: A highly trained, hard-working physician who has given most of your professional life to providing superb care. A plaintiff attorney is out to make you appear incompetent, and his motive is clear: He’ll earn one-quarter to one-third of any award that he wins for his client.
You are obviously convinced of the correctness of what you did in the case—or you wouldn’t have gone to court to defend yourself. You know the medicine better than the plaintiff lawyer does and, having been the caregiver, you can discuss all aspects of the case with much greater authority than he ever can. His only advantage? You’re in his backyard and he controls many of the rules.
But if you’re meticulously prepared, if you work with your lawyer and follow her advice, and if you are aware of the plaintiff attorneys’ tricks and techniques that I’ve described, you can neutralize much of the disadvantage you’re under in the legal system and defend your case on a greatly leveled playing field.
The author reports that he is president of Shoulder Dystocia Litigation Consultants, working with defense lawyers, insurance company case managers, and hospital risk managers in shoulder dystocia-related injuries and litigation.
CASE
You are a defendant in a malpractice case, and your lawyer has just finished questioning you—the “direct” part of your testimony. She asked you straightforward questions and you answered fully and without interruption. You were able to explain, at length, your account of what happened during the events in question. This is the first time you’ve been sued; you’re nervous, but things have gone well so far, you feel.
Cross-examination by the plaintiff’s attorney comes next. He starts aggressively, questioning the quality of your training and experience. Have any disciplinary actions ever been taken against you by your hospital or the state licensing board? Did you have specialty fellowship training? He makes it seem that, if you didn’t, you have no business taking care of patients.
He drills in: Have you taken courses in the specific area at question in the case—as if whole courses are given routinely on the narrow topics that are often the subject of litigation, whether shoulder dystocia, placental abruption, damage to a ureter, or other bad outcomes.
He moves on to ask about details of the case but cuts you off when you try to flesh out your answers. He admonishes you: Listen to the question and answer “Yes” or “No”!
He begins to raise his voice.
The attorney attacks your notes in the medical record; he makes them seem incomplete and inadequate. He tells members of the jury that they can assume that you did not take a specific action, despite your claim to the contrary, because it’s not in the record: “If it wasn’t written down, it didn’t happen.”
His demeanor becomes more confrontational. The increasingly abusive questioning goes on and on, and your sense that things are going well has evaporated.
How, you ask yourself as the assault continues, did all this rancor and accusation come on so fast and so unexpectedly?
This scenario, or versions close to it, occurs all too often to physicians in courtrooms across the United States. Defendant physicians who are vilified and goaded feel angry, frustrated, and helpless. No wonder—the courtroom environment is alien to us. We trained for years to become competent, knowledgeable practitioners of our specialty; we work hard every day to provide the best possible care; and we diligently keep up with advances in ObGyn medicine by reading the literature and attending continuing medical education conferences. But in the courtroom, attorneys make a pointed attempt to paint us as incompetent and uncaring—even malicious.
Moreover, customary rules of argumentation don’t apply. We can’t answer questions fully or correct misstatements that are implicit in certain questions. Judges often limit what we can say and what the jury is allowed to hear. Not only is the medical care we gave questioned—we are subject to attempts to discredit us personally. We’re asked questions about the most private aspects of our life: “What’s your income?” “Why were you divorced?” “What is the financial arrangement between you and your partners?” “Are you seeing—have you ever seen—a psychiatrist?”
The playing field has been set at a tilt
Perhaps your greatest disadvantage when you are sued is that, most likely, this is going to be your first time in a courtroom. You haven’t had the chance to become familiar with the venue—the courtroom—or the tactics of cross-examination used by plaintiff attorneys.
Combine an accusation of malpractice and the need to defend yourself in an alien environment with rules made by and favoring lawyers that are foreign to you and that you cannot control—what a daunting prospect! Plaintiff attorneys take advantage of the situation to prey on defendants.
There are ways to defend yourself!
Did you go into an operating room or a delivery room for the first time without preparation or training? No! Likewise, don’t go into a courtroom unprepared.
You may be surprised to learn that you do have advantages over lawyers for plaintiffs:
- You know more medicine than they ever will, no matter how many malpractice cases they have tried.
- You were there when the actions under dispute took place. You can speak from direct experience about those actions, with authority, as a knowledgeable eyewitness.
- Despite how it may appear, you have the right to defend your actions and your statements vigorously.
Plaintiff lawyers routinely employ a standard repertoire of tricks and traps, which I have seen used time and again. My goals here are to describe them to you so that you can see them coming and to tell you how to defend yourself against them. You’ll then be in a position to counter these tricks by 1) giving them a name, 2) confronting the lawyer—in front of the judge and the jury—with what he or she is attempting to do, and 3) employing defensive tactics.
A note about language in this article: For simplicity, when I say “he” when referring to a physician or lawyer, I mean “he” or “she.” And I mean “plaintiff attorney” when I say just “lawyer” or “attorney,” unless I am referring explicitly to your (the defendant’s) representation.
First, three little words to set the stage
Always keep in mind that, for you to be found guilty of malpractice, the plaintiff attorney has to prove beyond a reasonable doubt that the actions you did, or did not, take violated what is known in the medicolegal arena as standard of care. Because this standard is what you are being judged against, it is vital that you understand—and, in turn, that the jury understands—exactly what the term means.
Standard of care is defined as care generally given by well-trained physicians in your own specialty under similar circumstances. Standard of care does not mean “ideal” care, as may be recommended in a medical textbook or other kinds of professional communication. The standard of care is, essentially, generally accepted practice: The level and degree of care most often used by your contemporary peers. You are guilty of malpractice only if the care that you gave fell below the care that would generally have been given to a patient by others, in your specialty, under the circumstances you faced.
Inside an attorney’s bulging bag of tricks
What tactics might an attorney use to harass and intimidate you?
He’ll bully you. Imagine this: A plaintiff lawyer is brought into a surgical suite for the first time. He is asked to participate in an operation but isn’t allowed to speak unless spoken to. He is allowed to answer direct questions only in a format dictated by the senior surgeon. That lawyer would not know what was going on, would be continuously on the defensive, and would feel totally in over his head—if he didn’t faint first!
What I just described is the equivalent of what happens to you in a courtroom. An attorney is allowed great leeway over the types of questions that he can ask and the manner in which they can be posed. He often attempts to intimidate you with harsh language, a raised voice, physical gestures, and sarcasm. He might ask questions with implied premises that aren’t true. His behavior might be confrontational. He might try to cut you off. And he might insist that your answers be solely “Yes” or “No.”
He’ll troll through your CV. Every educational activity in which you have participated, and every professional position you have ever held, is subject to inquiry. In addition to being asked if you have ever been sued or had disciplinary action taken against you, an attorney will review your education, step by step. He might imply that, if you were educated abroad or went to a less-than-well-known medical school, you are poorly trained or somehow not “of high quality.” He will likely ask you how many times you took the specialty board exam before you passed it. You might even be asked how high you finished in your medical school class, or if you were given your first choice of residency program in a match.
He’ll create artificial standards in the minds of jury members. You might be asked if you have published in your field or if you have an academic appointment—the assumption being that, if your answer is “No,” your opinion about issues being discussed at the trial are not as authoritative as (he will claim) those of the plaintiff’s expert witness, who may be well known in the specialty.
He’ll take statements out of context. Articles that you published (even if years ago), previous depositions or trial testimony you have given, and even PowerPoint presentations you made to nurses on your labor and delivery unit may be probed and quoted. Usually, the attorney presents only brief snippets of these works, which are likely to be read to the jury out of context.
He’ll ask for specific references. Often, when an attorney asks about facts that you’ve mentioned or opinions you hold regarding issues that bear on the case in your trial, he will attempt to embarrass you by asking you to name the specific text, article, or author from which you obtained that information. Here’s an example: You know that the threshold for macrosomia in a shoulder dystocia case is 4,500 g, and that random late decelerations in a fetal monitor strip marked by otherwise excellent variability do not demand immediate C-section—but you may not be able to cite, off the top of your head, exactly in which textbook or journal article you read this or the information can be found. You might also be asked what an ACOG Bulletin or your hospital’s policy book says about a certain subject or aspect of care.
He’ll drag in the medical record and informed consent. An attorney might try to convince a jury that “if it isn’t written down, it didn’t happen.” He might cite a lack of an extensive written description of what occurred during the events in question as evidence of sloppy charting or poor care. He might claim that lack of a detailed note replicating a conversation that took place during the consent process displays a lack of concern for the patient’s right to know.
He’ll imply the existence of a standard of care. Lawyers often try to convince a jury that a defendant physician committed malpractice by claiming that she should have taken certain actions, when, in fact, these actions would have been unnecessary or inappropriate under the circumstances. Examples: Asking whether clinical pelvimetry was documented in the chart of a multiparous woman who came in actively laboring, or asking if fundal height was measured in the office during a patient’s last three prenatal visits.
Here are two other examples:
- In a case involving vacuum extraction delivery: “Doctor, have you ever read the vacuum device’s product safety manual?”
- When a plaintiff has testified that she told you at her first prenatal visit that her previous pregnancies were uncomplicated: “Did you call for, or read, the record from any of her previous pregnancies?”
He’ll create a false impression. A common attorney’s tactic is to pose questions to you that imply that certain things are true, when they are not. A common example of this tactic occurs in shoulder dystocia cases, when putative risk factors are addressed.
Consensus in the shoulder dystocia literature is that there are only three or four statistically consistent risk factors for this condition: shoulder dystocia in a prior delivery, macrosomia, gestational diabetes, and (possibly) mid-vacuum or forceps delivery. Often, however, attorneys imply to the jury that many other risk factors exist—and that your patient had any number of them and that you should have been aware of them.
You might be asked if your patient underwent oxytocin induction, had a long first stage of labor, had an epidural anesthetic placed, or was post-dates—none of which have a proven association with shoulder dystocia. You’ll be given little leeway, in answering questions posed to you, to try to refute the lawyer’s false assumptions. The impression may thus be left by this concatenation of nonproven factors that your patient was at high risk of shoulder dystocia, that this was foreseeable, and that you were negligent in not having performed a C-section to prevent it.
Likewise, lawyers often deliberately misuse statistics—such as when they discuss sonographic variability in the estimation of fetal weight: “Don’t you acknowledge, Doctor, that ultrasound estimates of fetal weight can vary by 15% of the actual weight? So why didn’t you take into account that the 4,300 g estimate you were given could, in fact, have been as high as 4,700 g?” Given the rules that restrict how you can answer, you are rarely allowed to explain to a jury that, first, the 15% variability applies only to a baby whose weight is more than one standard deviation from average and, second, the weight-estimate variability can be on the low side as well as on the high side.
How should you respond to interrogation?
Although you face disadvantages as a defendant physician in a courtroom, there are ways to fight back—to stick up for yourself and respond to the techniques that attorneys perpetrate. You aren’t as defenseless as it might appear!
Never allow an attorney to bully you in the courtroom or at a deposition. If the attorney begins to use such behavior, call it by its name and demand that it be stopped. Your lawyer will likely have raised an objection before you do; if she does not, protest such inappropriate behavior yourself. Never allow an attorney who is questioning you to raise his voice or speak to you sarcastically or rudely.
You don’t necessarily have to play by the rules for answering questions, despite any admonition by a plaintiff lawyer that you do so. Unless you are advised otherwise by the judge or by your lawyer, answer questions the way you want to, as long as your answer is a reply to the question that was asked. You are never obliged to answer a question with just “Yes” or “No.” If an attorney tries to impose such a limitation on you, declare that you cannot answer the question under those terms. If your answers are being cut off, don’t hesitate to tell the jury that you are not being allowed to tell the whole story.
If questions posed to you contain false premises, point that out. For example, you might be asked, “Given the obvious fetal distress that was present, why did you apply forceps?” If there was no fetal distress, or if that is one of the issues in dispute, you can respond that the question contains incorrect information or an unwarranted assumption, and therefore cannot be answered as asked.
Prepare to be asked about your background and training. Have your lawyer ask you preemptively, during her questioning, about anything in your professional life that might appear the least bit negative. This allows you to explain the matter fully without being cut off by the plaintiff attorney. Have your lawyer ask questions that show how your background and training compare with those of other physicians in your hospital and community. If you have been sued in the past, have your lawyer ask you about how many times an ObGyn is sued, on average, in her career (“three” is the answer), and use this fact to show the jury that being sued is not an anomaly but the rule in ObGyn practice.
Never answer a question about something you wrote in the past or about prior testimony without demanding to read it yourself, on the stand, in context. (The same is true for quotations from the medical literature read to you by the plaintiff attorney: You have a right to know the source and date of publication of quoted material, and you should insist on being able to read the quotation for yourself so that you can understand it in context.)
If asked from what text or article you learned a specific piece of information, point out the absurdity of being asked to remember such specifics from among the tens of thousands of things you have learned and read over your training and career.
When asked about your notes in the medical record and why you did or did not write a particular item, point out that the medical record is not a document that is intended to be used to prosecute or defend medical cases years down the road but rather is meant to convey important clinical information among health-care providers. Tell the jury what sorts of notes are routinely written and how much information is generally put into a note. If the notes you wrote are appropriate, even if brief, be sure and explain to the jury that what you did is, in fact, the standard of care—not an idealized conception taken from a textbook or an expert’s talk as to how notes should be written. Don’t agree with a lawyer’s contention that “if it isn’t written down, it didn’t happen.” That may be a lawyer’s rule; it is not a medical rule. Do not let the jury go into the jury room thinking that it is.
Know the specifics of your case. It is true that, as a defendant witness, several factors are out of your control. But don’t forget what you do have under your control: Knowledge of obstetrics and gynecology and experience in the field. You know the medical issues involved in the litigation better than anyone else in the courtroom.
Still, do your homework. Make sure that you know the specifics of your case, inside and out. Study the medical record of the case carefully and read all the depositions your lawyer provides for you. Know what the relevant ACOG Bulletin, major texts (such as Williams Obstetrics), and the literature say about the issues that are involved. Know who the experts are in this area of care and be prepared to quote pertinent articles that they have written. Work to never let yourself be surprised by the facts of the case or the medical information presented by the plaintiff’s side. Treat your testimony as a very important final examination. Do that, and you will be in an excellent position to successfully answer questions and refute incorrect statements.
Preempt questions about informed consent. Ask your lawyer to have you explain, during the direct portion of your testimony, about informed consent conversations, how they are usually held, and how they are documented. Tell the jury the difference between a calm consent discussion in the office before a routine medical procedure and a consent discussion in an urgent situation. By the way: The general rule about informed consent is that a physician is obliged to discuss with a patient any significant risk greater than 1%. This is a documented standard.1
Don’t let incorrect claims go unchallenged. Consider this scenario: A plaintiff attorney states that, given the circumstances of a certain clinical situation, you should have taken a particular action. This is often the case in fetal asphyxia cases, when experts for the plaintiff often testify that they can tell, from looking at the fetal heart rate monitoring strip, the exact moment at which a fetus was in trouble and should have been delivered by C-section. Consider having your lawyer issue an in-court challenge to an expert witness who makes such a claim to perform a blind reading of five fetal monitor strips for which the outcomes are known and to see if his predictions are correct. A plaintiff attorney will never take you up on such a challenge—and that refusal will be noted and appreciated by the jury.
This isn’t your backyard but you can play here
Amid what is often hostile treatment, it can be difficult to remember who you are: A highly trained, hard-working physician who has given most of your professional life to providing superb care. A plaintiff attorney is out to make you appear incompetent, and his motive is clear: He’ll earn one-quarter to one-third of any award that he wins for his client.
You are obviously convinced of the correctness of what you did in the case—or you wouldn’t have gone to court to defend yourself. You know the medicine better than the plaintiff lawyer does and, having been the caregiver, you can discuss all aspects of the case with much greater authority than he ever can. His only advantage? You’re in his backyard and he controls many of the rules.
But if you’re meticulously prepared, if you work with your lawyer and follow her advice, and if you are aware of the plaintiff attorneys’ tricks and techniques that I’ve described, you can neutralize much of the disadvantage you’re under in the legal system and defend your case on a greatly leveled playing field.
Reference
1. Nichols DL, Caldwell JW. Medicolegal complications consequent to unauthorized surgery. In: Nichols DH, DeLancey JOL, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1995:445-447.
Reference
1. Nichols DL, Caldwell JW. Medicolegal complications consequent to unauthorized surgery. In: Nichols DH, DeLancey JOL, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. 3rd ed. Baltimore, Md: Williams & Wilkins; 1995:445-447.
Shoulder dystocia: What is the legal standard of care?
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
If permanent injury occurs after shoulder dystocia, it can also trigger a lawsuit that can last for years and end in a large jury verdict—even if you handled the case with textbook perfection. Lawsuits involving brachial plexus injuries following shoulder dystocia are now the second most common type of lawsuit in obstetrics, exceeded only by those due to neurologic damage from birth asphyxia.1 Brachial plexus injury is often difficult to defend in court and results in scores of millions of dollars in damages each year. The plaintiff is usually a lovely child with an obvious and permanent injury, and the defense is typically an undocumented claim that the obstetrician applied no undue force at delivery.(Sidebar)
Given the difficulties of knowing when shoulder dystocia will occur, how best to resolve it, and whether a claim is likely, how can we prepare for this event? What is the accepted standard of care? This article answers these questions by surveying the evidence on these aspects of management:
- risk factors for shoulder dystocia
- how to choose mode of delivery
- specific labor-management practices
- the 4 most widely used maneuvers to resolve shoulder dystocia
- what information the documentation should include.
No single “standard of care”
In many states, the term “standard of care” has a specific legal meaning, but in most of the United States—and to most physicians— the term means care that would be rendered by the majority of well-trained individuals. Complicating this definition is the fact that medicine often offers no single “right way.” Thus, it may be more appropriate to speak of “standards of care”: the range of therapeutic choices a reasonable practitioner might decide to use.
Traction is the most used and abused of terms in shoulder dystocia lawsuits. Many plaintiff expert witnesses claim that traction should never be applied to a baby’s head during delivery. Other “experts” claim only “gentle” traction is warranted. These statements are designed to support the most frequent contention against obstetricians when permanent brachial plexus injury occurs: As there is an injury, it must have been caused by a doctor or midwife who used “excessive traction” to deliver the baby. This statement is usually made without defining “excessive” and without evidence that more force than necessary was used.
“Excessive” vs “minimum necessary” traction
Routine or “moderate” traction is used in most deliveries. The birth attendant almost always depresses the fetal head and applies a moderate amount of traction to it to help the baby’s anterior shoulder slide beneath the mother’s pubic bone.38 The only time traction is unnecessary is when the expulsive forces of the mother are so strong or uncontrolled that she pushes the baby out entirely on her own.
There is ambiguity—often contrived—about what exactly constitutes mild, moderate, routine, and “excessive” traction. No study has ever been published that accurately and unambiguously quantifies the amount of force used in actual deliveries.
Once shoulder dystocia is diagnosed, further attempts at routine traction without the use of other maneuvers should be avoided. At best these attempts are unavailing. At worst they serve only to keep the anterior shoulder lodged behind the maternal symphysis.
Much misinformation surrounds the role of traction during the McRoberts maneuver and other efforts to resolve dystocia. The reality is simple: An obstetrician cannot determine whether a maneuver has released the anterior shoulder unless moderate traction is applied after the maneuver to see if the baby can be delivered. Although extreme force at this or any point is not appropriate, moderate traction is entirely appropriate.
“Excessive traction” is an oxymoron, although plaintiff lawyers often use the term. An obstetrician uses a given amount of force in attempting to free a stuck shoulder. Once the shoulder is freed, no more force is applied. Thus, by definition, “excessive force”—more force than is necessary to deliver the baby—is never used. The proper term to describe the amount of force applied by a physician to resolve shoulder dystocia is “minimum necessary traction.”
Injury can follow a traction-free delivery
For many years, obstetricians familiar with shoulder dystocia have claimed that brachial plexus injuries can occur even in the absence of significant traction—either in utero or as a result of the natural forces of labor. Yet plaintiff attorneys and expert witnesses have contended that all brachial plexus injuries are the result of someone pulling “too hard.”
A recent case reported by Allen and Gurewitsch39 settled this question once and for all. They describe a delivery in which a patient requested no intervention of any kind. Despite no hand having touched the baby during delivery—thus, no “excessive traction” having been applied —the baby suffered a brachial plexus injury. This case proved that brachial plexus injuries can occur spontaneously and are not necessarily caused by traction.
Why dystocia cannot be predicted
…despite known risk factors
The risk of shoulder dystocia is higher in women with diabetes,2-5 a macrosomic fetus,2,6-8 obesity,5,8 or a previous shoulder dystocia.9-11 The problem: The predictive value of these factors is so low and their false-positive rate so high they cannot be used reliably in clinical decision-making.11-13
Prevention is impossible
Even if prediction were possible, the only preventive option is elective cesarean section. After all, this is the only intervention that might potentially avoid the infrequent but dreaded outcomes of asphyxia and permanent brachial plexus injury. But as the literature shows, even this is not an absolute guarantee.14,15 Moreover, the strategy of inducing labor several weeks prior to the due date to prevent a baby from becoming “too big” has been shown in many studies to be ineffective in lowering the shoulder dystocia rate.16-18
Risk factors are not clinically useful
The American College of Obstetricians and Gynecologists (ACOG) and Williams Obstetrics concur that risk factors for shoulder dystocia cannot be applied in a clinically useful way to prevent brachial plexus injury. As the ACOG practice bulletin on shoulder dystocia19 observes:
- “Shoulder dystocia cannot be predicted or prevented because accurate methods for identifying which fetuses will experience this complication do not exist.”
- “Elective induction of labor or elective cesarean delivery for all women suspected of carrying a fetus with macrosomia is not appropriate.”
Identify highest risk
Nevertheless, there are generally accepted guidelines for attempting to ascertain which patients are at the absolute highest risk for shoulder dystocia:
- Any woman with gestational diabetes. For any given week of gestation in the third trimester, the ratio of thorax and shoulder size to head volume is larger in babies of diabetic mothers.20 Thus, in these women, it is important to estimate fetal weight near term to determine whether a trial of vaginal delivery makes sense.
- If, for any reason, the fetus appears to be larger than average. Indications of size may come from palpation of the maternal abdomen, fundal height measurements significantly greater than dates, ultrasound estimation of large fetal weight, or maternal perception. In these cases, ultrasound imaging is advisable near term to estimate fetal weight. This estimate can be factored into the selection of delivery mode.
How big is “too big”?
There are 2 problems with using estimates of fetal weight in determining mothers and babies at highest risk:
- How is “too big” defined?
- What action should one take if a baby is thought to be “too big”?
As for what to do if a fetus is estimated to be in this size range, ACOG states: “Planned cesarean delivery to prevent shoulder dystocia may be considered [emphasis added] for suspected fetal macrosomia within the above weight parameters.”19 The decision as to whether to recommend or perform a cesarean section in these circumstances is intentionally left up to the physician and the patient.
The problem, of course, is that all our data are from measurements of babies after delivery—information obstetricians do not have at the time they must decide on the mode of delivery.
TABLE
How fetal weight affects the rate of dystocia
| ESTIMATED FETAL WEIGHT | RATE OF SHOULDER DYSTOCIA (%) | |
|---|---|---|
| NONDIABETIC MOTHERS | DIABETIC MOTHERS | |
| 1.1 | 3.7 | |
| 4,000–4,499 g | 10 | 23.1 |
| >5,000 g | 22.6 | 50 |
| Source: Acker D et al2 | ||
Choosing a mode of delivery: Not so simple
The obstetrician must determine whether the risk of shoulder dystocia is high enough to outweigh the risks to a mother of elective cesarean section. This is far from simple. Although it is true that women at the highest risk for dystocia—those with gestational diabetes and suspected macrosomia— have a risk for shoulder dystocia somewhere between 25% and 50%, this is not the main concern.
The main concern is this: What percentage of even these high-risk patients will have a shoulder dystocia that results in a permanent brachial plexus injury? The answer: Permanent injury is rare, even in highest-risk cases.
Only 10% to 20% of infants born after shoulder dystocia suffer brachial plexus injuries.16,21-23 Of these, only 10% to 15% are permanently injured.5,24,25 Thus, even in women at highest risk, the odds of having an infant with permanent brachial plexus injury are roughly 1 in 450.14 In women at lower risk for shoulder dystocia, the odds of permanent brachial plexus injury are much lower: somewhere between 1 in 2,500 and 1 in 10,000.
When is cesarean section warranted?
In deciding the answer to this question, the obstetrician must consider that cesarean section is not without its own risks: excessive bleeding, infection, injury to bowel or bladder, deep venous thrombosis, and the need for hysterectomy.
These adverse events occur much more frequently than does permanent brachial plexus injury.26 And the risks are higher yet for the very same patients at greatest risk for shoulder dystocia—diabetic and obese women.
Prevent “I didn’t know” accusations
This is the point at which the patient’s input becomes vital. It is important to convey to her in readily understandable terms the risks—to both her and her child—of cesarean section versus attempted vaginal delivery. Plaintiff attorneys often claim that, had their client known there was a 1 in 450 chance of her baby having a permanent injury, she would have opted for cesarean section. The truth of this claim is, of course, open to question. However, from a medicolegal perspective, it is extremely important that the woman be informed of the degree of risk to herself and her baby so that her decision is truly informed—even if it is not the choice the obstetrician would have made.
The consensus in surgery is that the patient should be informed when the threshold of risk for an adverse event reaches 1% or higher. Although it is an informal teaching, this threshold is documented in the medical literature.27
The option of cesarean section should be discussed and possibly recommended for all women whose infants are estimated to weigh more than 5,000 g in the absence of diabetes and 4,500 g or more in women with diabetes.
Often a mother will voice concern about whether she will be able to deliver her baby safely vaginally. She may feel that her infant is too big, that she is too small, or that her obesity will make her delivery more difficult. Do not blithely ignore such concerns or provide blanket reassurances that everything will be OK.
Instead, review with her any risk factors she may have for shoulder dystocia and discuss the specific odds of injury to her baby should dystocia arise. Then discuss the risks to her and the discomfort she will experience if she elects a cesarean section.
Patients have a right to know the risks
Although it is appropriate to be reassuring when there are no significant risk factors, patients deserve to know what risks they run and to have these risks put into perspective. For example, if the mother has diabetes and her baby is estimated to weigh over 4,500 g, the risk of permanent brachial plexus injury approaches 1 in 450. The same is true if she is nondiabetic but has an estimated fetal weight of 5,000 g or more.
In high-risk cases such as these, you should discuss the risks with the patient and have her participate in the decisionmaking. You should also clearly document this discussion in the medical record.
Labor management
Prolonged second stage and instrumental delivery
Although the literature is not clear on this point, there is a trend toward increased rates of shoulder dystocia with a prolonged second stage of labor2,3,28 and with instrumental deliveries.6,12,29,30 Most experts believe this trend merely reflects the fact that bigger babies—the known major risk factor for shoulder dystocia—encounter these sorts of labor problems more frequently than do smaller babies. Whatever the reason, it warrants attention. An obstetrician’s care of any laboring woman should follow standard practices regarding arrest of labor and descent or a prolonged second stage.
Plaintiffs are quick to condemn vacuum and forceps
The same applies to intervention with forceps or vacuum. Only in women at highest risk for shoulder dystocia—those with diabetes or with suspected macrosomic fetuses—should standard management be modified.
Given the potential for shoulder dystocia in such high-risk circumstances, not to mention our inability to predict dystocia, prudence dictates that we avoid aggressive management and the use of forceps or vacuum in these cases.
These practices are often condemned in court by plaintiff lawyers and their expert witnesses.
Oxytocin is OK
In cases of arrest of labor and descent, the use of oxytocin is appropriate. A laboring woman should be given adequate time to deliver on her own, especially if a regional anesthetic has been used.
…but prepare to act quickly. In high-risk cases, be prepared to move more quickly than normal to cesarean section.
Is your team prepared? 4 standards of care
Although it is true that an obstetrician must be prepared for the possibility of shoulder dystocia in any delivery, to act as though it will occur in all deliveries is simply not reasonable, given that the rate of dystocia is 0.5% to 1.5%, or 1 in 67 to 200 deliveries.12,21,25,29
Nevertheless, 4 specific standards apply to all delivery facilities:
- The entire labor and delivery staff should know what to do and what each person’s role is when shoulder dystocia is diagnosed.
- Labor and delivery nurses should know how and when to initiate McRoberts maneuver and apply suprapubic pressure.
- The team should immediately obtain the assistance of another obstetrician, a pediatrician, and an anesthesiologist, even though they are not likely to arrive before the dystocia is resolved.
- The obstetrician should be mentally prepared for the possibility of shoulder dystocia. This requires the ability to quickly recognize it, familiarity with the various techniques for resolving it, and avoidance of unnecessary traction. It also is vital for the obstetrician to remain composed and in charge, as the obstetrician becomes the leader of the medical team when this emergency arises.
How to recognize shoulder dystocia
There are 2 ways to diagnose dystocia.
- “Turtle sign.” The first is recognizing the pathognomonic “turtle sign,” in which, after delivery of the baby’s head, the head immediately retracts back up against the mother’s perineum, causing the baby’s cheeks to bulge.
- The second diagnostic sign is when, after delivery of the head, the moderate amount of traction usually used does not suffice to deliver the anterior shoulder. Cease attempts at routine traction as soon as shoulder dystocia is diagnosed.
The 4 main maneuvers
The 4 maneuvers generally used by obstetricians to resolve shoulder dystocia are considered the standard of care:
- McRoberts maneuver
- Suprapubic pressure
- Woods screw maneuver
- Delivery of the posterior arm
McRoberts maneuver is often the only one needed
In this maneuver, the laboring woman’s thighs are hyperflexed against her abdomen.31 This hyperflexion does not increase the diameter of the pelvis, as is sometimes claimed. Rather, it flattens the sacrum and changes the angle of the symphysis pubis in relation to the baby’s anterior shoulder, often freeing it. It is an extremely effective way to resolve shoulder dystocia and is often the only maneuver necessary.
Family members can assist—contrary to plaintiff attorney contentions. This maneuver can be performed by nurses or family members if they are properly instructed. Plaintiff attorneys will sometimes argue that the use of family members in this situation is inappropriate, but they are wrong. Family members are sometimes instructed to hold a mother’s legs in a certain position while she is pushing; they can certainly be instructed to hold the legs against the maternal abdomen during attempts to resolve a shoulder dystocia.
Suprapubic pressure with or without McRoberts
In this maneuver, a nurse or other attendant places direct pressure with an open hand or fist just above the mother’s symphysis pubis. The pressure can be directed straight down or to the left or right. Wherever it is directed, the aim of the pressure is to push the baby’s anterior shoulder out of its position behind the mother’s pubic bone.
The combination of McRoberts maneuver and suprapubic pressure can resolve shoulder dystocia in as many as 58% of cases.22
Woods screw maneuver attempts to “spin” the baby
If the McRoberts maneuver and suprapubic pressure do not resolve the shoulder dystocia, the Woods screw maneuver is usually implemented next.32 In this maneuver, the obstetrician inserts a hand into the posterior vagina and pushes the front of the baby’s posterior shoulder in a spiral direction (clockwise or counterclockwise). The goal is to “unjam” the anterior shoulder from its trapped position behind the symphysis pubis.
The Woods screw maneuver is very effective. After it has been used, it is appropriate to apply moderate traction to the baby’s head to determine whether the baby can be delivered.
Variant: Rubens maneuver. In this maneuver, the obstetrician pushes on the posterior aspect of the posterior shoulder. In addition to spinning the shoulders, as in the Woods screw maneuver, the Rubens maneuver causes shoulder abduction, thus decreasing the biacromial diameter that has to pass through the pelvic outlet.
Attempts to deliver the posterior arm
If shoulder dystocia still persists, the next strategy is usually an attempt to deliver the baby’s posterior arm. This is done by placing a hand deep into the posterior aspect of the vagina, grabbing the baby’s posterior arm, sweeping that arm across the baby’s chest, and delivering it. Once the posterior arm and shoulder are delivered, it is almost always possible to deliver the baby directly from this position or to move the baby in a spiral direction (clockwise or counterclockwise) to free the anterior shoulder.
Other maneuvers
Two other maneuvers are occasionally used, though neither is considered mainstream.
Gaskin or “all fours” maneuver. This technique is frequently advocated by the midwife community.33 It involves moving the laboring woman from the standard lithotomy pushing position to her hands and knees to free the stuck anterior shoulder. However, many have questioned the practicality of turning a fatigued, laboring woman rapidly enough to deliver a baby within the 4 to 6 minutes available, particularly when an epidural has been given or other maneuvers have already used up much of the allotted time.
Zavanelli maneuver if all else fails. This maneuver should be attempted only when all other efforts have failed.34 It involves flexing the fetal head and attempting to push the baby’s head back into the vagina, followed by emergency cesarean section.
Although case reports have described successful use of this maneuver, there also have been reports of fetal death, fractured spines, and other severe fetal damage. Thus, this maneuver should be the absolute last resort in desperate emergencies.35
What not to do
Traction
Do not continue to apply traction to the fetal head if the shoulder does not come. Once shoulder dystocia is diagnosed, cease all attempts to deliver the baby by continued pulling. Carefully but expeditiously use the various maneuvers you were trained to do, applying moderate traction after each one to see if the shoulder has been freed.
Fundal pressure
Do not apply fundal pressure. It never helps resolve shoulder dystocia, but only further jams the stuck shoulder against the maternal pubic bone. It also can cause injury to the fetus or even rupture the uterus.
Fundal pressure is often cited in court as a definite standard of care violation.
Theory vs evidence
A 3-member team is adequate
Shoulder dystocia occurs unexpectedly. Once it does occur, the obstetrician has 4 to 6 minutes to resolve it before the threat of central neurologic damage to the baby becomes significant. Although it would be very helpful for additional personnel to be available, it is not always possible to assemble this team quickly enough.
In reality, the only personnel truly necessary to resolve a shoulder dystocia are:
- The delivering doctor or midwife
- A medically trained assistant familiar with McRoberts maneuver and suprapubic pressure
- Any other available person, including a family member, who can be drafted to help and instructed to participate in the McRoberts maneuver by flexing one of the mother’s thighs
Drills are not an absolute necessity
It is sometimes claimed that formal shoulder dystocia drills should be conducted in labor and delivery units at fixed intervals. Although this may be a useful and reasonable educational practice, it is more important that each individual on the labor and delivery team know what his or her role is during such an emergency. Whether this is achieved through a practice drill or didactic instruction does not matter.
In short, there is nothing about the concept of a drill that is “standard of care.” What is standard of care is that every team member knows what to do, how to do it, when to do it, and how to document it.
Episiotomy is often superfluous
Multiple studies have shown that episiotomy is not necessary to resolve shoulder dystocia, although many textbooks and other published protocols still recommend it.36 The obstructing factor in shoulder dystocia is not the soft tissue of the perineum but the symphysis pubis. The only time episiotomy helps is when more room is needed for the obstetrician’s hand to enter the posterior aspect of the vagina to perform a shoulder dystocia maneuver. If you can perform all necessary maneuvers without episiotomy, it is superfluous.
Document early and always
Because shoulder dystocia often leads to litigation, it is extremely important to document what happened during delivery as soon as feasible and in as much detail as possible. Standardized forms are now available. (FORM)
At minimum, you should record:
- how shoulder dystocia was diagnosed
- which shoulder was anterior and which was posterior
- quantification of the force applied initially and in subsequent traction attempts, using terms such as “mild,” “moderate,” or “significant”
- duration of attempts to resolve the dystocia
- maneuvers performed
- approximate length of time each maneuver was tried
- condition of the baby at delivery, including Apgar scores, a description of all injuries and bruises, and cord pH, if obtained
- time from delivery of the fetal head to delivery of the body
- documentation of the discussion with the patient following delivery
Given that the most frequent criticism of obstetricians in the courtroom in brachial plexus injury lawsuits is that they pulled too hard, the best defense consists of careful, complete, and contemporaneous documentation of one’s actions at delivery.
Lawsuits happen
Even when everything is done correctly, there is a very high likelihood that a lawsuit will be filed when there is a permanent brachial plexus injury.
The 2 claims generally made against obstetricians are:
- The obstetrician should have known or predicted that the risk of shoulder dystocia was high, and should have performed a cesarean section or at least offered the mother that choice.
- As the baby has a permanent brachial plexus injury, the obstetrician must have pulled too hard at delivery.
The best defense
The best defense is, as always, to have practiced good medicine and to have documented it. You must be able to demonstrate from your records—years after a delivery that you no longer remember—that you:
- made appropriate prenatal judgments and were aware of risk factors
- informed the mother of such risk factors when they are significant
- provided proper obstetrical care
- documented in the medical record that you knew what you were doing and did it correctly
Some good news is on the horizon. Recent research has produced a mathematical tool that appears to be able to predict 50% to 75% of all women destined to have shoulder dystocia, with a false-positive rate of only 2% to 3%.37 If this model holds up under further investigation, it may become possible to avoid most shoulder dystocia deliveries and, with them, permanent brachial plexus injuries.
Meanwhile, what is an obstetrician to do about shoulder dystocia? As always, give the best care you can. Know the risk factors. When possible, consider alternatives to vaginal delivery and be less aggressive in the management of labor. Know the techniques for resolving shoulder dystocia and have a preestablished plan for what to do.
Document, document, document.You can give the best care in the world, but if you cannot demonstrate on paper years down the road that you did so, our current liability system will make it seem as if you did not.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.
1. Professional Insurance Association of America risk management data, 2005.
2. Acker D, Sachs B, Friedman E. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
3. Al-Najashi S, Al-Suleiman S, El-Yahia A, Rahman M, Rahman J. Shoulder dystocia—a clinical study of 56 cases. Aust N Z J Obstet Gynaecol. 1989;29:129.-
4. Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873.
5. Sandmire HF, O’Halloin TJ. Shoulder dystocia: its incidence and associated risk factors. Int J Obstet Gynaecol. 1988;26:65-73.
6. Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:47-480.
7. Kolderup LB, Laros RK, Jr, Musci TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
8. Emerson R. Obesity and its association with the complications of pregnancy. Br Med J. 1962;2:516-519.
9. Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? Br J Obstet Gynaecol. 1994;101:713-715.
10. Ginsberg NA, Moisidis C. How to predict recurrent shoulder dystocia. Am J Obstet Gynecol. 2001;184:1427-1430.
11. Gherman RB. Shoulder dystocia: an evidence-based evaluation of the obstetrical nightmare. Clin Obstet Gynecol. 2002;45:345-361.
12. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:14-17.
13. Lewis DF, Edwards MS, Asrat T, et al. Can shoulder dystocia be predicted? Preconceptual and prenatal factors. J Reprod Med. 1998;43:654-658.
14. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480-1486.
15. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
16. Delpapa DH, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstet Gynecol. 1991;78:340-343.
17. Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol. 1997;89:913-917.
18. Leaphart WL, Meyer MC, Capeless EL. Labor induction with a prenatal diagnosis of fetal macrosomia. J Matern Fetal Med. 1997;6:99-102.
19. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #40: Shoulder Dystocia. Washington, DC: ACOG; November 2002.
20. Elliott JP, Garite TJ, Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159-162.
21. Gherman RB. Persistent brachial plexus injury: the outcome of concern among patients with suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.-
22. McFarland MB, Langer O, Piper JM, Berkus MD. Perinatal outcome and the type and number of maneuvers in shoulder dystocia. Int J Obstet Gynaecol. 1996;55:219-224.
23. Bofill JA, Rust OA, Devidas M, et al. Shoulder dystocia and operative vaginal delivery. J Matern Fetal Med. 1997;6:220-224.
24. Johnson NR. Shoulder dystocia: a study of 47 cases. Aust N Z J Obstet Gynaecol. 1979;19:28-31.
25. Nocon JJ, Weisbrod L. Shoulder dystocia. Chapter 14. In: O’Grady JP, Gimovsky M, eds. Operative Obstetrics. Philadelphia: Williams & Wilkins; 1995:339-353.
26. Creasy RK, Resnik R. Maternal-Fetal Medicine. 5th ed. Philadelphia: Saunders; 2004:690-691.
27. Nichols DH, DeLancey JO, eds. Clinical Problems, Injuries and Complications of Gynecologic and Obstetric Surgery. Baltimore: Williams & Wilkins; 1995:447.
28. Hopewood HG. Shoulder dystocia: fifteen years’ experience in a community hospital. Am J Obstet Gynecol. 1982;144:162-166.
29. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526-529.
30. McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784-788.
31. Gonik B, Stringer CA, Held B. An alternate mechanism for management of shoulder dystocia. Am J Obstet Gynecol. 1983;145:882-884.
32. Woods CE. A principle of physics as applicable to shoulder delivery. Am J Obstet Gynecol. 1943;45:796-804.
33. Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. J Reprod Med. 1998;43:439-443.
34. Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. Am J Obstet Gynecol. 1985;152:479.-
35. Sandberg EC. The Zavanelli maneuver: 12 years of recorded experience. Obstet Gynecol. 1999;93:312-317.
36. Gurewitsch ED, Donithan M, Stalllings SP, et al. Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes. Am J Obstet Gynecol. 2004;191:911-916.
37. Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14 [Epub ahead of print].
38. DeCherney AH, Pernoll ML, eds. Lange Obstetric and Gynecologic Diagnosis and Treatment. 8th ed. Norwalk, Conn: Appleton & Lange; 1994:219.
39. Allen RH, Gurewitsch ED. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet Gynecol. 2005;105:1210-1212.
Dr. Lerner is a consultant for LMS Medical Services.