User login
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
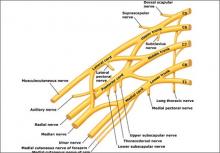
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.