User login
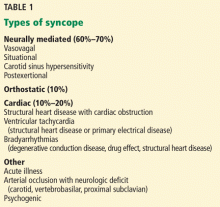
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
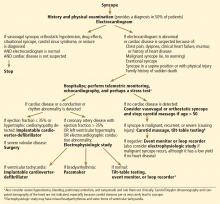
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
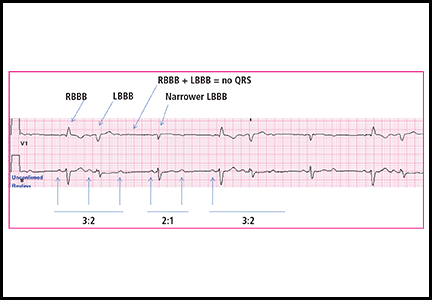
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
KEY POINTS
- Neurally mediated forms of syncope, such as vasovagal, result from autonomic reflexes that respond inappropriately, leading to vasodilation and relative bradycardia.
- Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction, volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
- The likelihood of cardiac syncope is low in patients with normal electrocardiographic and echocardiographic findings.
- Hospitalization is indicated in patients with syncope who have or are suspected of having structural heart disease.