User login
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
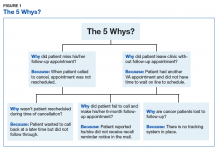
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
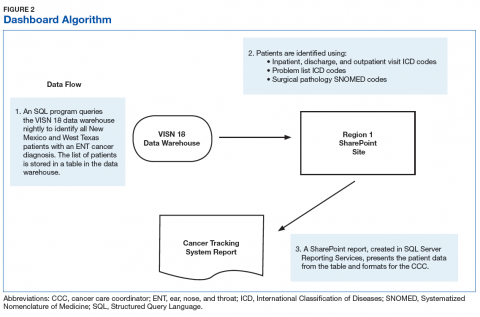
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
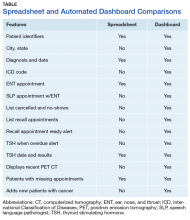
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.