User login
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.
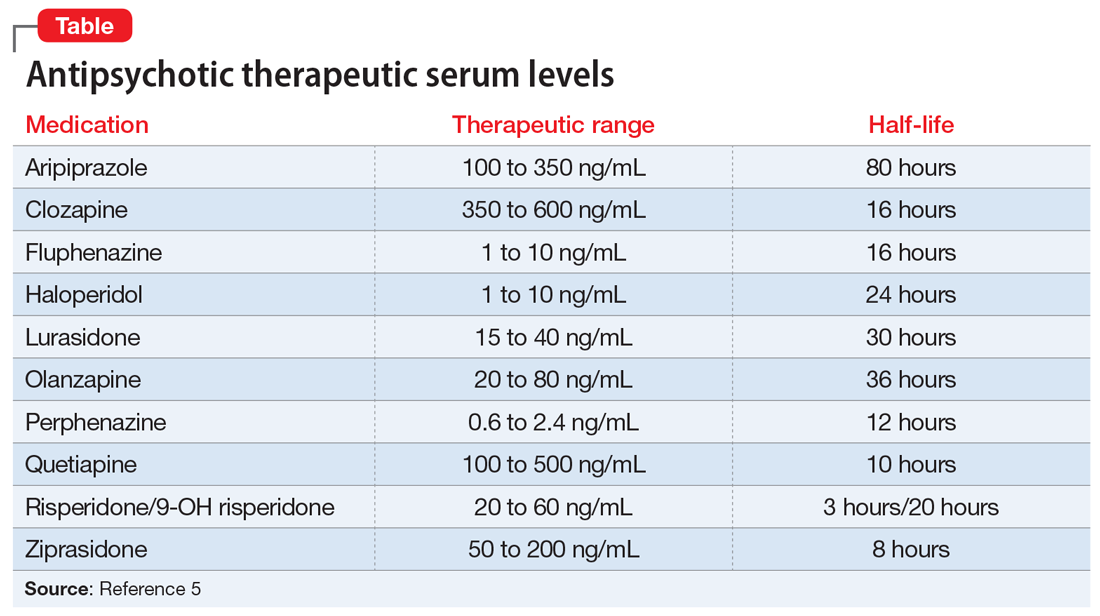
Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.

