User login
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
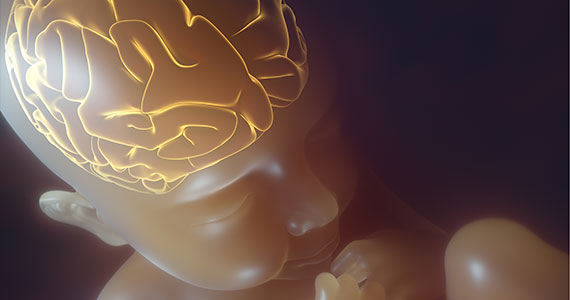
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7
Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
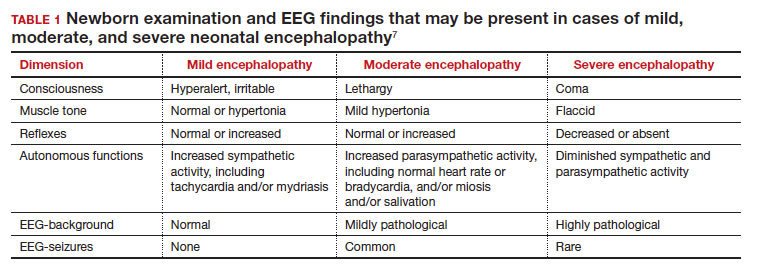
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.
Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.