User login
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
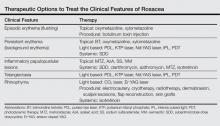
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
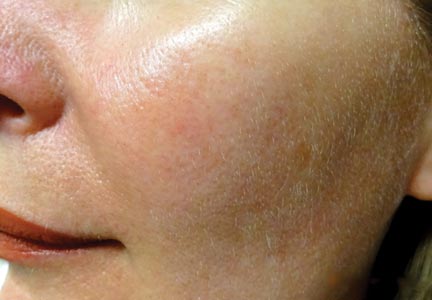
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
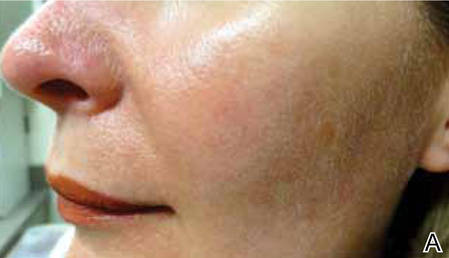 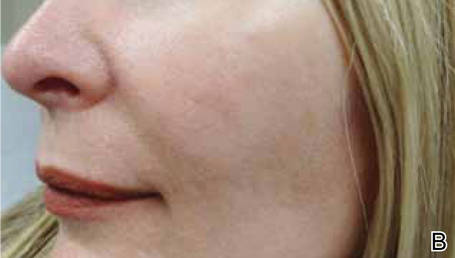 |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
Rosacea is a commonly encountered chronic inflammatory skin disease that affects an estimated 16 million Americans and exhibits a particular predilection for the convexities of the central face (eg, forehead, cheeks, nose, chin).1,2 The pathophysiology of rosacea remains poorly understood despite the relatively high prevalence of the disease and substantial ongoing research.3 The current paradigm suggests a complex multifactorial interplay involving aberrations of the innate and adaptive immune system, neurovascular dysregulation, blood and lymphatic vessel changes, genetic predispositions, and overgrowth of commensal organisms such as Demodex.3 Additionally, a variety of external factors may exacerbate clinical symptoms (eg, UV radiation, heat exposure, spicy food, alcohol, stress).
The diagnosis of rosacea is made clinically and rarely requires histologic confirmation. Although rosacea can present with a wide range of clinical features that often wax and wane over time, a near universal finding is diffuse centrofacial erythema.4 This centrofacial redness may symptomatically worsen during a flare period, causing flushing, but it often persists nontransiently between flares as background erythema. Other variable findings of rosacea include the presence of telangiectases, edema, plaques, phymatous changes, dry skin, ocular manifestations, and inflammatory lesions in the form of papules and pustules.5 Patients also may report a stinging or burning sensation in affected areas. It is important to note that most patients will only exhibit some of these clinical features and that symptoms often vary in the timing of their emergence or regression.5 A classification system has been developed for rosacea that categorizes the disease into 4 subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) and one variant (granulomatous).6 These categories are determined by the grouping of clinical features present, but it is not uncommon for patients to exhibit clinical manifestations of more than 1 subtype.7
The detrimental cosmetic effects of rosacea are obvious given its chronic nature and tendency to affect highly visible areas such as the face. As such, rosacea can have a devastating impact on patients’ quality of life.8 Patients with rosacea have been reported to have higher incidence rates of low self-esteem, embarrassment, social anxiety, and depression as compared to the rest of the population. Effective treatment, however, can improve cosmetic appearance and mitigate the negative psychosocial impacts of the disease.8
Treatment of rosacea focuses on relieving cosmetic symptoms, as no curative therapy currently exists. Treatment comes in a wide variety of forms, including topical medications, systemic pharmacologic therapies, light-based modalities, and procedural interventions. Choice of therapy should be determined on a case-by-case basis as guided by the clinical features present, and combination or sequential therapies often are required to achieve optimal cosmetic results. In this article, we review both existing and emerging treatments of rosacea and assess their ability to improve the cosmetic symptoms of rosacea (Table).
Skin Care
Proper skin care is an important aspect of treatment for all patients with rosacea and thus includes the use of over-the-counter cleansers, moisturizers, and sunscreens.9 The choice of skin care products is an important consideration given the often hypersensitive skin of rosacea patients. Moisturizers and cleansers should have an acidic to neutral pH, similar to normal skin. They should not contain emulsifiers that strip moisture from the skin or protective lipids and proteins from the stratum corneum.10 Moisturizers without irritants, abrasives, or allergens should be used following skin cleansing. Protection from UV radiation with sunscreen, ideally with a sun protection factor greater than 30, is particularly important, as it can prevent UV-induced rosacea flares as well as photodamage that can cause additional erythema and telangiectasia.4 Rosacea patients also may find green-tinted makeup to be useful in concealing areas of erythema.8
Topical Therapy
Currently, there are only 5 US Food and Drug Administration (FDA)–approved topical medications for the treatment of rosacea: metronidazole (MTZ) gel 0.75% and 1%, azelaic acid (AzA) gel 15%, sodium sulfacetamide (SS) 10%–sulfur 5% lotion and cream, brimonidine tartrate (BT) gel 0.5%, and the most recently approved ivermectin (IVM) cream 1%.7 Metronidazole, AzA, and SS primarily are used to treat the inflammatory papules and pustules of rosacea, while BT is used to treat persistent background erythema. The exact mechanisms of action by which MTZ, AzA, and SS treat rosacea are unclear, but they are thought to reduce inflammation and/or immune response. Metronidazole and AzA both have demonstrated favorable safety profiles and significant (P<.05) efficacy over vehicle in reducing inflammatory lesions in numerous well-controlled randomized clinical studies.4,11,12 There is some evidence that AzA may be more effective than MTZ; one 15-week multicenter, double-blind, randomized, parallel-group study demonstrated that twice-daily AzA gel 15% showed significant superiority (P=.02) over twice-daily MTZ gel 0.75% in improving the inflammatory lesions and erythema of rosacea.13 Sodium sulfacetamide also has shown good efficacy in the treatment of inflammatory lesions and performed significantly better (P=.04) than MTZ according to one multicenter, investigator-blinded, randomized, parallel-group study,14 but the overall evidence is not as strong as MTZ and AzA.4,11,15 The most common adverse effect for MTZ, AzA, and SS is application-site irritation, but overall most patients report good tolerance to these topical medications.4 Azelaic acid is unique in that patients may report stinging, tingling, or burning after application, but these effects are not associated with visible skin changes and usually are transient, generally remitting after 1 to 2 weeks.4
Brimonidine tartrate is a highly selective α2-adrenergic receptor agonist whose mechanism of action in the treatment of rosacea is thought to involve vasoconstriction of superficial skin vasculature and to a lesser extent anti-inflammatory effects.16 In a double-blind, randomized, vehicle-controlled phase 3 trial, application of BT gel 0.5% once daily for 4 weeks demonstrated significant efficacy over vehicle (P<.001) in treating persistent nontransient facial erythema in 553 adult patients with 2 or fewer papulopustular lesions as evaluated over 12 hours on days 1, 15, and 29.17 Notably, a substantial difference in cosmetic appearance was observed in another study as early as 30 minutes after the first gel application on day 1.18 The results of this phase 3 trial17 mirrored those of the phase 2 dose-optimization and safety studies of similar design.18 In addition to another long-term, 1-year, open-label study,19 both phase 2 and 3 studies have shown favorable safety profiles with no reports of tachyphylaxis, rebound erythema, or aggravation of other disease features such as telangiectases or inflammatory lesions.17,18 Recently, however, there have been some reports of considerable rebound erythema with BT use and thus patients should be made aware of this possibility.20,21 Case reports of successful treatment of background erythema and flushing with other topically applied adrenergic receptor modifiers such as oxymetazoline and xylometazoline have been published in the literature,22,23 but additional research will be necessary to validate these claims.
Ivermectin, a decades-old antiparasitic, has recently shown promising results as a treatment of rosacea patients with moderate to severe papulopustular lesions. Its therapeutic effect is believed to be mediated by its activity against Demodex, a natural skin mite that has been found at increased concentrations in a subset of patients with rosacea, as well as by its natural anti-inflammatory properties.24 In 2 identically designed, randomized, double-blind, controlled trials of IVM cream 1% applied once daily for 12 weeks, a significantly larger proportion of patients in the IVM groups achieved an investigator global assessment of clear or almost clear as compared to vehicle (IVM: 38.4% and 40.1%, respectively; vehicle: 11.6% and 18.8%, respectively; P<.001). Both trials also demonstrated that IVM was significantly superior to vehicle in the reduction of inflammatory lesion counts measured at week 12 as compared to baseline (IVM: 76.0% and 75.0%, respectively; vehicle: 50.0% and 50.0%, respectively; P<.001).24 An extension of these original trials demonstrated long-term safety with up to 52 weeks of topical IVM use and reported a low incidence rate of adverse effects, most commonly transient skin burning, pruritus, and dryness. Notably, the incidence rate of these adverse effects was lower than a comparison group receiving AzA gel 15% once daily.25 Once-daily application of IVM cream 1% also has recently demonstrated superiority over twice-daily MTZ cream 0.75% for 16 weeks in a phase 3 investigator-blinded, randomized, parallel-group study. The IVM group was significantly superior to MTZ in the reduction of inflammatory lesions as compared to baseline (83.0% vs 73.7%) and in the number of participants who achieved an investigator global assessment score of clear or almost clear (84.9% vs 75.4%)(both P<.001).26 There also is limited evidence for the use of other antiparasitic topical medications such as crotamiton 10% and permethrin 5%, but such agents frequently cause irritation and may not be well tolerated in rosacea patients.27-29
There are a variety of other non–FDA-approved topical medications that have been used with varying success in the literature, including cyclosporine, macrolides, benzoyl peroxide, retinoids, and calcineurin inhibitors such as tacrolimus and pimecrolimus. Evidence for the use of these medications generally is limited to a few studies with small numbers of patients and will not be discussed further in this article.4,11,30 These agents, however, may be useful in select cases when first-line regimens have failed and also may be good targets for future research.
Systemic Therapy
The mainstay of systemic treatment of rosacea centers around the tetracyclines, a group of antibiotics that have been used off label for rosacea since the 1950s.31 The therapeutic effects of tetracyclines in the treatment of rosacea are thought to revolve around their anti-inflammatory effects rather than their antibacterial properties.32 Currently, the only FDA-approved oral agent for treatment of the inflammatory lesions of rosacea is doxycycline 40-mg modified-release capsules taken once daily. These modified capsules allow for instant release of 30 mg and delayed release of 10 mg of doxycycline. This dosing is considered to be anti-inflammatory rather than antimicrobial, as it does not produce antibiotic selection pressure even with prolonged use.33 Efficacy of 40-mg subantimicrobial-dose doxycycline (SDD) has been demonstrated in 2 phase 3 multicenter, parallel-group, randomized, double-blind, placebo-controlled studies in which SDD demonstrated a significantly greater reduction in the number of total inflammatory lesions at week 16 compared to placebo (P<.001).34 Subantimicrobial-dose doxycycline also has been shown to be equally as efficacious in reducing inflammatory lesions as traditional-dose doxycycline.35 There also is some evidence for the efficacy of SDD in reducing overall erythema, as demonstrated by one open-label, community-based study in which SDD monotherapy resulted in clinician erythema assessment scores of mild or no erythema in 75% of patients with mild to severe rosacea at baseline after 12 weeks of therapy.35 Additionally, SDD is considered to be safe and well-tolerated and does not generally result in the adverse effects that may be seen in antibiotic-level doses of doxycycline (eg, gastrointestinal upset, vaginal candidiasis, photosensitivity).34,36,37 Other antibiotics such as clarithromycin, azithromycin, and MTZ also have been studied as treatments of papulopustular rosacea at antibiotic-level doses with good therapeutic effect.38-40 These therapies, however, generally are not used unless there are contraindications for use of tetracycline antibiotics, such as pregnancy or allergy, as the overall evidence is not as strong and there may be increased risks for serious adverse effects.30
Although it is not FDA approved, isotretinoin is an important therapeutic option for select rosacea patients, as it is the only pharmacologic agent that has shown efficacy for the phymatous changes of rosacea. Its efficacy, however, is limited to early-stage rhinophyma that has not yet progressed to the fibrotic or mucinous stages of disease in which it has been shown to reduce the size and number of cutaneous sebaceous glands.30,41 Isotretinoin at 0.3 mg/kg daily also has shown noninferiority in treatment of the inflammatory papules and pustules of rosacea as compared to antibiotic dosing of doxycycline in one large-scale, placebo-controlled, randomized, 12-week multicenter study.42 Unfortunately, recurrence is highly likely after isotretinoin therapy is discontinued.30,41 However, continuous “microdose” isotretinoin at 0.03 to 0.17 mg/kg daily has shown evidence for efficacy in treatment of recalcitrant papulopustular disease.43 Such dosing may have the added benefit of reduced risk for radiographic changes associated with long-term isotretinoin use.43
Light-Based Therapy
Light-based modalities are an important tool set in the management of rosacea symptoms, as they can treat telangiectases for which medical therapy is not generally effective.9 To a lesser extent, light-based modalities also can help alleviate background erythema. The most commonly used light-based modalities include the pulsed dye laser (PDL)(Figure), potassium titanyl phosphate (KTP) laser, Nd:YAG laser, intense pulsed light, photodynamic therapy, CO2 laser, and erbium-doped YAG (Er:YAG) laser. These treatments produce clinical results by targeting specific chromophores such as oxyhemoglobin, deoxyhemoglobin, methemoglobin, and clotted blood with light of specific wavelengths to induce thermolysis of vasculature while sparing collateral tissue.44 Generally, larger telangiectatic vessels are more amenable to therapy than smaller vessels, which usually require higher energy to be delivered in a shorter period of time, thus predisposing the patient to the development of purpura that may last for 1 to 2 weeks.44
  |
Historically, PDL used a light wavelength of 577 nm and was classically associated with posttherapy purpura; however, modern PDLs use wavelengths of 585 or 595 nm and are associated with a reduced risk for purpura through the use of longer pulse durations (ie, 10–40 millisecond), multiple minipulses, multiple passes, and advanced epidermal cooling methods.9,44 In a small, prospective, randomized, controlled, nonblinded study, PDL therapy with fluence sufficiently high enough to induce purpura achieved an approximate 50% improvement in telangiectasia grading scores in most patients after a single treatment.45 Notably, PDL therapy at purpura-inducing settings was reported to be much more efficacious than settings that did not induce purpura (purpura free), especially in the treatment of thicker telangiectases.45
Potassium titanyl phosphate lasers make use of shorter wavelengths (532 nm) than PDL and thus are better able to target superficial vasculature, which translates into a reduced risk for purpura and faster healing times. However, KTP laser therapy typically is only reserved for patients with lighter skin types, as this wavelength of light is more likely to result in higher melanin absorption and possible postinflammatory hyperpigmentation.44 A split-face study comparing the KTP laser with PDL determined that the KTP laser was able to achieve 62% clearing after the first treatment and 85% clearance after the third treatment versus 49% and 75% for PDL treatment, respectively; however, the KTP laser had higher rates of posttherapy erythema lasting at least 1 day (58% vs 8%).46
Conversely, the Nd:YAG laser uses longer wavelengths (1064 nm) and can achieve deeper skin penetration, which may be effective for larger, recalcitrant, or deeper blue-tinted vessels. A split-face, double-blind, randomized, controlled trial found Nd:YAG laser therapy to be an effective treatment of facial erythema, though it was observed to be less effective than purpura-free PDL therapy in reducing redness after 4 treatments (34% vs 52% improvement, respectively); however, treatment with the Nd:YAG laser was found to be significantly (P=.0028) less painful.47
Intense pulsed light is unique from the previously discussed light-based therapies in that it uses noncoherent light with wavelengths between 500 and 1200 nm. Cutoff filters may be used to allow for more selective tissue damage depending on the depth of penetration desired. Intense pulsed light has been shown to be equally as efficacious as purpura-free PDL therapy in the treatment of erythema and telangiectasia in a randomized, controlled, single-blind, split-face trial.48 Additionally, a study of 200 patients with facial vascular lesions, of whom 74 patients had rosacea, showed that intense pulsed light therapy resulted in a 75% to 100% improvement of lesions in 174 of 188 (92.5%) patients who returned for follow-up. Treatment often required at least 2 sessions, but overall adverse effects were reported to be minimal.49
Photodynamic therapy is a well-studied and often utilized treatment of a variety of skin conditions, but there have only been a few studies regarding its use in rosacea. Photodynamic therapy involves the use of topically applied photosensitizing agents such as 5-aminolevulinic acid or methyl aminolevulinate before exposure to red or blue light. This process generates reactive oxygen species, though the exact mechanism of action through which patients achieve cosmetic improvement in rosacea is unclear. In one study of 17 patients with varying rosacea subtypes treated with methyl aminolevulinate and red light, drastic relief of symptoms was seen in 10 (58.8%) patients, marked improvement in 4 (23.5%) patients, and no response in 3 (17.6%) patients. Most patients report a transient irritant skin reaction at the site of therapy.50
Ablative lasers such as the CO2 (10,600 nm) and Er:YAG (2940 nm) lasers also have been shown to be useful in the treatment of rosacea, specifically for the management of rhinophymatous features. Excellent results have been achieved with these lasers given their ability to provide near-bloodless surgical fields. In a 13-year review of 124 patients with rhinophyma receiving a single CO2 laser treatment, good to excellent results were achieved in 118 (95.2%) of patients when evaluated at 3 months posttreatment.51 Patient satisfaction also is reported to be high with few adverse effects reported. The evidence for the Er:YAG laser is not as strong, but the current reports indicate efficacy and safety similar to that of the CO2 laser.52
Procedural Therapies
Procedural therapies in rosacea generally are reserved for management of rhinophyma and include electrocautery, cryotherapy, radiotherapy, dermabrasion, scalpel excisions, flap reconstruction, and skin grafts.30,53 The details and evidence for these methods is beyond the scope of this paper, but it is important to be aware of such modalities. As with most surgical procedures, operator skill and experience may affect treatment outcomes, and there also are definite risks for postprocedural scarring, swelling, erythema, and pigmentation changes. Recently, anecdotal evidence has shown that botulinum toxin injections may be effective for patients with refractory flushing and erythema, but larger studies will be necessary to better assess these claims.54,55
Conclusion
Although recent advances in pharmacology and laser technology have provided physicians with new and effective treatment modalities for rosacea, it remains a poorly understood disease without a definitive cure. The negative impact of rosacea on patients’ quality of life can be substantial, but effective management of cosmetic symptoms can minimize such deleterious effects. Therapy should be individualized and directed at treating the symptoms that are most bothersome to the patient. Additionally, effective treatment often will require a combination of modalities or sequential therapies to achieve optimal cosmetic outcomes.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
1. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
2. Rosacea prevalence map. National Rosacea Society Web site. http://rosacea.org/press/prevalencemap. Accessed June 16, 2015.
3. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
4. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
5. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
6. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
7. Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20:623-629.
8. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
9. Mansouri Y, Goldenberg G. Devices and topical agents for rosacea management. Cutis. 2014;94:21-25.
10. Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
11. van Zuuren EJ, Kramer S, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2011;3:CD003262.
12. Liu RH, Smith MK, Basta SA, et al. Azelaic acid in the treatment of papulopustular rosacea: a systematic review of randomized controlled trials. Arch Dermatol. 2006;142:1047-1052.
13. Elewski B, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
14. Torok HM, Webster G, Dunlap FE, et al. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis. 2005;75:357-363.
15. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
16. Piwnica D, Rosignoli C, de Menonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective alpha-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49-54.
17. Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
18. Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0.5% is a novel treatment of moderate to severe facial erythema of rosacea: results of two multicenter, randomized and vehicle-controlled studies. Br J Dermatol. 2012;166:633-641.
19. Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13:56-61.
20. Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:e37-e38.
21. Ilkovitch D, Pomerantz RG. Brimonidine effective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70:e109-e110.
22. Kim JH, Oh YS, Ji JH, et al. Rosacea (erythematotelangiectatic type) effectively improved by topical xylometazoline. J Dermatol. 2011;38:510-513.
23. Shanler SD, Ondo AL. Successful treatment of erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor antagonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
24. Stein-Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13:316-323.
25. Stein-Gold L, Kircik L, Fowler J, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13:1380-1386.
26. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103-1110.
27. Koçak M, Ya˘gli S, Vahapo˘glu G, et al. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. a randomized double-blind placebo-controlled study. Dermatology (Basel). 2002;205:265-270.
28. Bikowski JB, Del Rosso JQ. Demodex dermatitis: a retrospective analysis of clinical diagnosis and successful treatment with topical crotamiton. J Clin Aesthet Dermatol. 2009;2:20-25.
29. Layton A, Thiboutot D. Emerging therapies in rosacea. J Am Acad Dermatol. 2013;69(6 suppl 1):S57-S65.
30. Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512, quiz 513-514.
31. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
32. Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287-294.
33. Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472-1483.
34. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791-802.
35. Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(suppl 5):7-15.
36. Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
37. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93:18-28.
38. Torresani C. Clarithromycin: a new perspective in rosacea treatment. Int J Dermatol. 1998;37:347-349.
39. Bakar O, Demircay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154.
40. Saihan EM, Burton JL. A double-blind trial of metronidazole versus oxytetracycline therapy for rosacea. Br J Dermatol. 1980;102:443-445.
41. Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011;4:54-61.
42. Gollnick H, Blume-Peytavi U, Szabo EL, et al. Systemic isotretinoin in the treatment of rosacea—doxycycline-and placebo-controlled, randomized clinical study. J Dtsch Dermatol Ges. 2010;8:505-515.
43. Hofer T. Continuous “microdose” isotretinoin in adult recalcitrant rosacea. Clin Exp Dermatol. 2004;29:204-205.
44. Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
45. Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
46. Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
47. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
48. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35:920-928.
49. Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
50. Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
51. Maden V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161:814-818.
52. Fincher EF, Gladstone HB. Use of a dual-mode erbium:YAG laser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6:267-271.
53. Lloyd KM. Surgical correction of rhinophyma. Arch Dermatol. 1990;126:721-723.
54. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-e79.
55. Park KY, Hyun MY, Jeong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
Practice Points
- As no definitive cure for rosacea exists, effective treatment is aimed at improving the cosmetic symptoms.
- Choice of therapy should be determined on a case-by-case basis as guided by the clinical features most bothersome to the patient.
- A combination of modalities and/or sequential therapy often is required to achieve optimal cosmetic outcomes.