User login

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
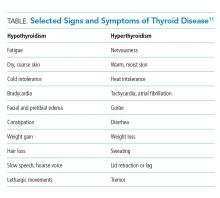
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
© 2020 Society of Hospital Medicine