User login
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
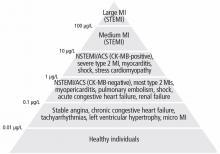
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
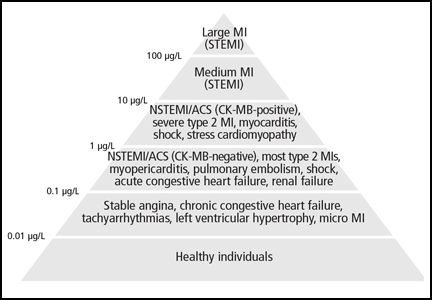
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
KEY POINTS
- Because newer assays for troponin can detect this biomarker at lower concentrations than earlier ones could, they are more sensitive but less specific.
- The high sensitivity of troponin assays makes them valuable for ruling out MI, but less so for ruling it in. Therefore, additional signs are required for the diagnosis.
- MI is categorized into several types, depending on whether it is spontaneous (acute coronary syndromes), caused by supply-demand mismatch, associated with sudden cardiac death, or a complication of percutaneous coronary intervention or of coronary artery bypass grafting.
- In settings in which nonspecific troponin elevations are frequently seen, a less sensitive but more specific test such as creatine kinase MB or troponin using a higher threshold value may be useful.