User login
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).
 |
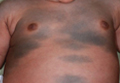
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |
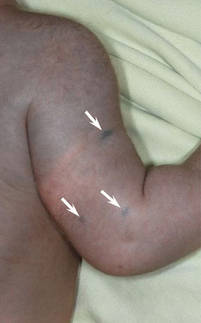 |
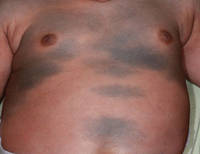
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |
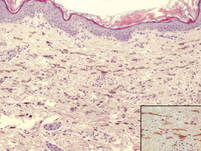 |
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).
 |
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |
 |
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |
 |
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).
 |
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |
 |
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |
 |
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.