User login
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.
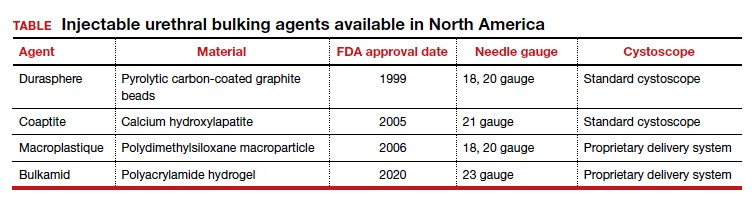
Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
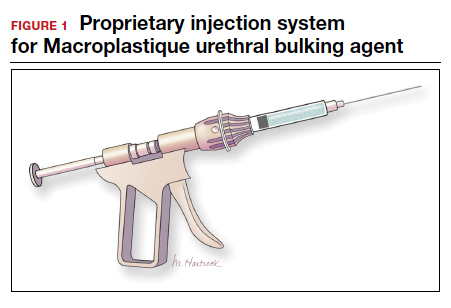
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4

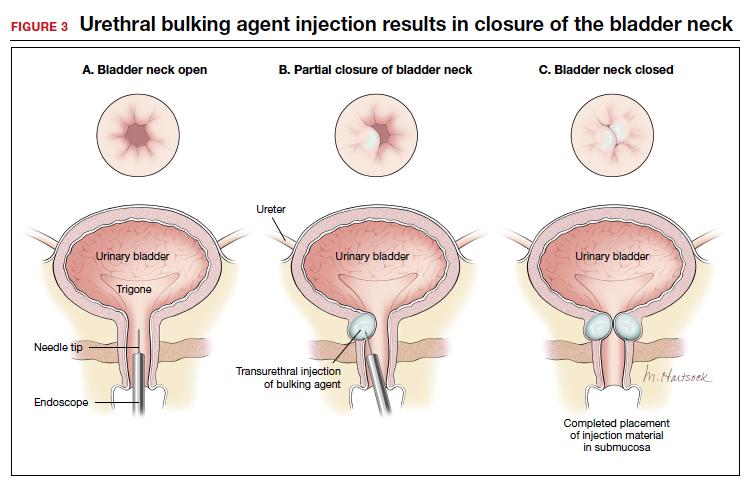
After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).
Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.

