User login
Testosterone supplementation in women: When, why, and how
There are no currently US Food and Drug Administration (FDA)-approved therapies for testosterone use in women. Its use by clinicians is through dose modification of FDA-approved therapies for men, or preparations created by compounding pharmacies. Recently, several professional organizations, including the American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society, International Society for the Study of Women’s Sexual Health, and the International Society for Sexual Medicine, convened an expert panel to develop a global position statement on testosterone therapy for women.1 In this roundtable for OBG Management, moderated by Mickey Karram, MD, several experts discuss this position statement as well as the overall clinical advantages and drawbacks of using testosterone in women.
Testosterone indications
Mickey Karram, MD: For which indications do you prescribe testosterone supplementation in women?
Lauren Streicher, MD: I offer systemic testosterone therapy to postmenopausal women who have hypoactive sexual desire disorder (HSDD) and low serum testosterone levels, with one caveat—it is important that the patient’s reported distressing lack of libido is not explained by another condition or circumstance. Many women present reporting low libido but, on further questioning, it is typically revealed that dyspareunia precipitated their loss of interest in sex. It is normal to not want to do something that is painful. In addition, low libido can often be explained by chronic disease, such as diabetes, cancer, or clinical depression.
Some medications, including selective serotonin reuptake inhibitors (SSRIs), frequently cause a decline in sexual interest. Finally, psychosocial and partner issues may be the culprit.
James Simon, MD, CCP, NCMP, IF: Much of the beneficial data for testosterone’s use is for sexual function in postmenopausal women.2 Female sexual dysfunction is highly prevalent among women during the postmenopause.3 Androgen levels progressively decrease throughout adult life in all women, so the postmenopausal additional lack of estrogen has a recognized effect on genitourinary health. There is evidence that the insufficiency of androgens as well as estrogens after menopause can lead to genitourinary symptoms of menopause (GSM).4
Testosterone also is used for increasing strength, lean muscle mass, bone mineral density, and sense of well-being.5
Rebecca Glaser, MD: I consider testosterone supplementation in my clinical practice in both premenopausal and postmenopausal women for symptoms of androgen/hormone deficiency, including diminished sense of well-being; dysphoric mood; anxiety; irritability; fatigue; decreased libido, sexual activity, or pleasure; vasomotor instability; bone loss; decreased muscle strength; insomnia; changes in cognition; memory loss; urinary symptoms; incontinence; vaginal atrophy and dryness; and joint and muscular pain. We also have shown through preliminary and short-term data and case studies that testosterone therapy has a potential beneficial effect on migraine headaches, as well as active breast cancers in both premenopausal and postmenopausal women.6-10
Continue to: What is appropriate bloodwork?...
What is appropriate bloodwork?
Dr. Karram: Do you obtain blood work before initiating testosterone treatment? If so, what tests do you order and what testosterone levels are considered to be normal for premenopausal and postmenopausal women?
Dr. Streicher: Unlike estrogen, which is predictably low in a postmenopausal woman, serum testosterone (T) levels are highly variable because of the adrenal component. Ovarian testosterone production does not cease at the same time as estrogen production. So I do obtain total and free T levels, prior to initiating treatment. Having said that, it has been well established that T levels correlate poorly with level of sexual interest, and there is no specific blood level that can be used to differentiate women with and without sexual dysfunction. We all have patients who have nonexistent T levels and have a very healthy libido, and other women with sky-high levels who have no libido. But it is useful to know levels prior to initiating therapy to be able to monitor levels throughout treatment. Also, if levels are in the premenopausal physiologic range, not only is she unlikely to respond but she is also at risk for developing androgenic adverse effects, such as acne and hair growth. In general, a low free T level (even if it is in the normal postmenopausal range) in a clinical setting of HSDD supports supplementation.
The assessment and interpretation of T levels can be challenging, particularly as the majority of testosterone is protein-bound and biologically inactive. Free T levels (the biologically active testosterone) in many labs are unreliable and need to be calculated.
In addition to total and free T, I check levels of sex hormone-binding globulin (SHBG), the protein that binds testosterone and renders it biologically inactive. If someone has high SHBG levels and is taking an oral estrogen, simply switching to a transdermal estrogen will result in decreased SHBG and increased free T.
Levels of total and free T vary from lab to lab, so it is best to be familiar with those ranges and then be consistent in which lab you choose.
Dr. Glaser: Although I personally do order blood work on most patients (T, free T, estradiol, complete blood count, thyroid-stimulating hormone, and follicle-stimulating hormone), after 15 years of research and publishing data on testosterone implants, I do not believe that T levels are absolutely necessary or even beneficial in most cases. It rarely changes management in my patients.
As Lauren said, it is well known that T levels do not correlate with androgen deficiency symptoms or clinical conditions caused by androgen deficiency. If a patient has symptoms of androgen deficiency, a trial of testosterone therapy should be given.
T levels are not a valid marker of tissue exposure in women, reflecting less than 20% of total androgen activity. The major source of testosterone in pre and postmenopausal women is the local intracrine production of testosterone from the adrenal precursor steroids dehydroepiandrosterone (DHEA) and androstenedione, which would not be reflected in T levels.
In our study involving 300 women, we found no relationship between baseline T levels, presenting symptoms, or response to therapy.6 Premenopausal and postmenopausal women had similar baseline T levels and similar response to therapy. Even women with baseline T levels in the mid-range responded to therapy.
Some of the most controversial topics in treating women with testosterone are related to dosing and T levels throughout therapy. Guideline authors often use the terms ‘physiologic dosing’ and ‘physiologic ranges’ when making recommendations for therapy. Although “physiologic” sounds appropriate/ scientific, these rigid opinions/recommendations are not evidence based. There are no data supporting the use of endogenous T ranges to guide dosing or monitor testosterone therapy.
The decision to initiate testosterone therapy is a clinical decision between the doctor and the patient based on the patient’s symptomatology, which is the therapeutic endpoint. Testosterone therapy must be done with adequate doses determined by clinical effect (benefits) versus side effects or adverse events (risks). T levels may be helpful, along with clinical evaluation when troubleshooting.
Utilizing data from thousands of patients, we have developed serum ranges for testosterone implants.11 Even so, no two patients are the same, nor do they respond to therapy the same. It is always a clinical decision.
Continue to: Dr. Simon...
Dr. Simon: In the recent global consensus statement on testosterone use,1 the experts were in agreement that “no cut-off blood level can be used for any measured circulating androgen to differentiate women with and without sexual dysfunction.” They give their recommendation a C, and I agree that testosterone supplementation, with specific dosage levels, are a clinical decision.
Before initiating testosterone therapy, it is recommended that liver function and fasting lipids are assessed, as liver disease and hyperlipidemia are contraindications to treatment. These levels should be monitored twice in the first year and annually thereafter while the patient is taking testosterone. Breast and pelvic examinations, mammography, and evaluation for abnormal bleeding should be performed as well as the blood tests.12 These recommendations are focused on safety not efficacy.
Administration route
Dr. Karram: How do you administer testosterone, and why?
Dr. Streicher: As there are no FDA approved testosterone products for women, clinicians must determine the dosage and route of delivery based on published clinical trials.
Dr. Glaser: I treat patients with subcutaneous pellet implants. The implants provide consistent and continuous delivery of therapeutic amounts of testosterone. There is a reason testosterone pellets have been used for more than 80 years and are more popular now than ever—they work. The insertion procedure is simple and takes about 2 minutes. The treatment is cost-effective, avoids first pass, has no adverse effect on the liver or clotting factors, and there is no transference. Decades of data support both the efficacy and safety of testosterone implants.6 However, testosterone implants are not regulated by the FDA and all patients are required to sign a consent informing them of off-label use, benefits, and risks of testosterone implant therapy.
Dr. Simon: I think the consent is important, as there is no package labeling to warn of possible side effects.
Dr. Streicher: Oral testosterone therapy, because of its first pass through the liver and association with adverse lipid profiles with negative effects on high- and low-density lipoprotein cholesterol levels, is not recommended. I prefer a transdermal approach. Pellets, implants, and injections have the potential to result in supraphysiologic blood concentrations. It must be emphasized that the goal of treatment is to approximate premenopausal physiologic levels. More is not better; excessive levels do not demonstrate a greater sexual response and are in fact more likely to have a negative impact due to androgenic side effects.
In most clinical trials, a 300 mg/d testosterone patch was effective, but these patches are not commercially available so I rely on transdermal gel from a compounding pharmacy. The typical dose needed to raise levels into the high to normal range for most women is 2.5 mg up to 5 mg per day of testosterone 1%, which translates to roughly 1 mL. Many pharmacies provide a dispenser, which allots the appropriate dose. Alternatively, I instruct the patient to place a dollop on her thigh (roughly in size of a single M&M candy).
I always tell my patients that the response is not immediate, typically taking 8 to 12 weeks for the effect to become clinically significant. I generally see a patient back 8 weeks after initiation of treatment to check T levels and evaluate response.
Dr. Simon: There are some data demonstrating that intravaginal testosterone can be a potential treatment for GSM. Intravaginal testosterone coupled with aromatase inhibitor therapy used for breast cancer treatment resulted in supraphysiologic T levels and reportedly improved vaginal maturation index and reduced dyspareunia. More study is needed.13
Dr. Streicher: Agreed. The lower third of the vagina and the vestibule is rich in testosterone receptors. Like Dr. Simon, in some cases of vaginal atrophy I prescribe a compounded local vaginal testosterone.
Continue to: Testosterone and premenopausal women...
Testosterone and premenopausal women
Dr. Karram: Is there a role for testosterone supplementation in premenopausal women with normal estrogen production?
Dr. Glaser: Yes. In fact, in our study, more than one-third of the patients were premenopausal, which makes sense.6 There is a marked decline of T levels and the adrenal precursor steroids (DHEA and androstenedione) in women between the ages of 20–30 years and around age 50. As we said, symptoms of androgen deficiency often occur prior to menopause and are not related to estrogen levels. In our study, testosterone implant therapy relieved symptoms of hormone (androgen) deficiency, including vasomotor symptoms, sleep problems, depressive mood, irritability, anxiety, physical and mental exhaustion (fatigue, memory issues), sexual problems, bladder problems (incontinence, frequency), vaginal dryness, and joint and muscular pain. Premenopausal and postmenopausal patients reported similar hormone deficiency symptoms. Premenopausal women did report a higher incidence of psychological complaints (depressive mood, anxiety, and irritability), while postmenopausal women reported more hot flashes, vaginal dryness, and urologic symptoms. Both groups demonstrated similar improvement in symptoms.
In addition, we have seen relief of severe migraine headache in premenopausal (as well as postmenopausal) women treated with testosterone implant therapy.6,7
Dr. Streicher: The goal of testosterone supplementation is to approximate physiological testosterone concentrations for premenopausal women. While testosterone may improve well-being and sexual function in premenopausal women, the data are limited and really inconclusive. More study is needed given that there is likely a wide therapeutic range with many variables. Having said that, there are some data that indicate that testosterone in premenopausal women may enhance general sense of well-being.14
Why is there no FDA-approved agent?
Dr. Karram: Why do you think the FDA has been reluctant to approve a testosterone agent for women?
Dr. Simon: Three potential testosterone drugs for use in women have been unsuccessfully brought to market after the FDA did not approve them. There are 31 approved products for men, each of which were approved because they safely restored normal testosterone concentrations in men with reduced levels and an associated medical condition. Unlike this scenario for men, for women, the FDA has required products to show clinical effectiveness in trials. For instance, Estratest, a combination estrogen-testosterone product, was in use in the 1960s—approved for women with estrogen-resistant hot flushes, and used in practice for sexual dysfunction. After the FDA implemented its Drug Efficacy Study and Implementation regulation system after 2000, which required safety and efficacy trial(s) before drug approval, the manufacturer removed the drug from market when presented efficacy study data for the added testosterone in the drug were deemed inadequate.15
Dr. Streicher: We have yet another example of the disparity between the FDA approval processes for sexual function drugs for men versus women. Take Intrinsia as another example. It was a 300-mg testosterone patch that underwent clinical trials in women who were post-oophorectomy with HSDD. The patch had demonstrated efficacy with minimal adverse effects and no statistically significant dangerous effects. However, the FDA declined approval, citing “safety considerations” and requested longer-term clinical trials to evaluate potential cardiovascular or breast problems. Given that Intrinsia supplementation simply restored normal physiologic testosterone levels, and there was no such requirement in men who received supplementation post-orchiectomy, this requirement was nonsensical and unjustified.
Compounded formulations
Dr. Karram: Are compounding pharmacies appropriately regulated, and how can you be assured that the source of your testosterone is appropriate?
Dr. Glaser: Compounding pharmacies are regulated by the State Boards of Pharmacy, Drug Enforcement Agency, Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, State Bureaus of Narcotics and Dangerous Drugs, and Departments of Health (in some states).
Compounding is a highly regulated profession that is constantly under scrutiny by agencies, patients, and physicians. Any additional regulations could adversely impact the accessibility of patients to individually compounded medications including intravenous and oncology medications. Over the past 20 years, I have treated hundreds of patients with breast cancer with compounded vaginal testosterone (with or without estriol) and subcutaneous testosterone (with or without anastrozole), greatly improving quality of life in women suffering from severe symptoms. Without the availability of compounded medications, there would have been no or limited alternatives for adequate and much needed therapy. Notably, there have been no adverse events or safety-related issues in more than 20 years.
Regarding whether or not “the source of your testosterone is appropriate,” pharmacists can only use United States Pharmacopeia (USP) grades of testosterone. Testosterone used in compounding is required by the FDA to be of USP grade from an FDA registered and compliant facility. In addition, compounding support companies run additional USP tests to confirm their products meet USP standards prior to being delivered to individual compounding pharmacies.
Dr. Streicher: However, there potentially can be substantial variability between formulations and batches. Product purity can also be an issue. It is reassuring if the compounding pharmacy is compliant with purity of Active Pharmaceutical Ingredients and Good Manufacturing Practice rules and guidelines that assure the minimum requirements to assure high quality and batch-to-batch consistency. I find it helpful to always work with the same pharmacy once you have established uniformity and reliability. If there is concern, it is appropriate to check a patient’s serum level 2 weeks after initiation of therapy.
Dr. Simon: I think the problem with some compounding pharmacies is that there may be incentives back and forth with the clinician to use a certain outlet, whereby the patient’s best interest may not be served. I do believe that there is a role for compounding pharmacies, however. We also use them because some women may have strange reactions or be allergic to the preservatives, formulating agents, or even lactose, in various pills and patches, gels, and creams.
Continue to: Testosterone for aging and cognition?...
Testosterone for aging and cognition?
Dr. Karram: Do you think that testosterone supplementation in the elderly can have a positive impact on aging, Alzheimer disease, and dementia?
Dr. Streicher: The jury is still out on the cognitive effects of postmenopausal androgen supplementation. There is currently insufficient evidence to support the use of testosterone to enhance cognitive performance, or to delay cognitive decline. I prescribe testosterone only to treat HSDD, but I do tell my patient that she may possibly also benefit in terms of cognitive function, musculoskeletal parameters, and well-being. Large RCTs are needed in those areas to justify prescribing for those benefits alone.
Dr. Simon: I would say this is the place for future development, but where there is very likely to be a benefit is on sarcopenia.
Dr. Glaser: There is some evidence that testosterone is neuroprotective.16 In my clinical practice I have seen “self-reported” memory issues improved on therapy, often returning toward the end of the testosterone implant cycle. Adequate amounts of bioavailable testosterone at the androgen receptor are critical for optimal health, immune function, and disease prevention.
Dr. Karram: In conclusion, this expert panel agrees that testosterone supplementation is beneficial for sexual dysfunction in postmenopausal women, with also many other potential benefits that require further investigation. Route of administration preferred by Dr. Simon and Dr. Streicher is transdermal or a transvaginal cream. Dr. Glaser uses a subcutaneous pellet approach. Thank you all for an engaging and informative discussion. ●
Dr. Karram: How would you treat the following patient? She is 56, postmenopausal, and taking estrogen. She reports decreased libido, fatigue, lack of sleep, and lack of focus. Would you consider testosterone supplementation?
Dr. Simon: For her libido, yes. I would not give it to her for the fatigue if it were simply lack of sleep and without an associated medical condition. For her lack of focus, the testosterone could be beneficial. The central nervous system effects of testosterone are thought to be related to the conversion of testosterone to estrogen in the brain; if a person’s getting enough estrogen, they shouldn’t have lack of focus. Since some women may not want more estrogen, administering a little testosterone for libido also offers focus because it adds to the estrogen in the brain. If after giving her adequate amounts of testosterone her libido is not better in 8 weeks, it wasn’t a testosterone problem. If she does report improvement, however, I would keep her on the agent as long as she is healthy. But most 56-year-old women who already met the criteria for going on estrogen should be fine with testosterone.
If this same patient were not reporting low libido but did report lack of strength, energy, or well-being I also would say, “Sure, give testosterone a try.”
Dr. Glaser: I also would treat her with testosterone—with pellet implants. The dose would depend on her body weight. I usually start with an approximate dose of 1 mg of testosterone per pound of body weight. This amount of testosterone delivered continuously from the implant also supplies estradiol (via aromatization) locally at the cellular level.
I would treat her for as long as she chooses to continue testosterone therapy. There is no end- or stop-date where a person no longer benefits from therapy or adverse events occur. Testosterone does not increase the risk of breast cancer and it has a positive effect on many of the adverse signs and symptoms of aging, including mental and physical deterioration.
- Davis SR, Baber R, Panay N, et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. Climacteric. 2019;22:429-434.
- Islam RM, Bell RJ, Green S, et al. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Diabetes Endocrinol. 2019;S2213-8587:30189-30195.
- Simon JA, Davis SR, Althof SE, et al. Sexual well-being after menopause: an International Menopause Society White Paper. Climacteric. 2018;21:415-427.
- Traish AM, Vignozzi L, Simon JA, et al. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev. 2018;6:558-571.
- Panay N. British Menopause Society tools for clinicians: testosterone replacement in menopause. Post Reprod Health. 2019;25:40-42.
- Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas. 2011;68:355-361.
- Glaser R, Dimitrakakis C, Trimble N, et al. Testosterone pellet implants and migraine headaches: a pilot study. Maturitas. 2012;71:385-388.
- Glaser RL, York AE, Dimitrakakis C. Efficacy of subcutaneous testosterone on menopausal symptoms in breast cancer survivors. J Clin Oncol. 2014;32(suppl):109-109.
- Glaser RL, Dimitrakakis C. Rapid response of breast cancer to neoadjuvant intramammary testosterone-anastrozole therapy: neoadjuvant hormone therapy in breast cancer. Menopause. 2014;21:673.
- Glaser RL, York AE, Dimitrakakis C. Subcutaneous testosterone-letrozole therapy before and concurrent with neoadjuvant breast chemotherapy: clinical response and therapeutic implications. Menopause. 2017;24:859-864.
- Glaser R, Kalantaridou S, Dimitrakakis C, et al. Testosterone implants in women: pharmacological dosing for a physiologic effect. Maturitas. 2013;74:179-184.
- International Society for the Study of Women’s Sexual Health (ISSWSH) clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. In press.
- Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause. 2018;25:837-847.
- Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
- Simon JA, Kapner MD. The saga of testosterone for menopausal women at the Food and Drug Administration (FDA). J Sex Med. 2020;17:826-829.
- Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3: 980-992.
There are no currently US Food and Drug Administration (FDA)-approved therapies for testosterone use in women. Its use by clinicians is through dose modification of FDA-approved therapies for men, or preparations created by compounding pharmacies. Recently, several professional organizations, including the American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society, International Society for the Study of Women’s Sexual Health, and the International Society for Sexual Medicine, convened an expert panel to develop a global position statement on testosterone therapy for women.1 In this roundtable for OBG Management, moderated by Mickey Karram, MD, several experts discuss this position statement as well as the overall clinical advantages and drawbacks of using testosterone in women.
Testosterone indications
Mickey Karram, MD: For which indications do you prescribe testosterone supplementation in women?
Lauren Streicher, MD: I offer systemic testosterone therapy to postmenopausal women who have hypoactive sexual desire disorder (HSDD) and low serum testosterone levels, with one caveat—it is important that the patient’s reported distressing lack of libido is not explained by another condition or circumstance. Many women present reporting low libido but, on further questioning, it is typically revealed that dyspareunia precipitated their loss of interest in sex. It is normal to not want to do something that is painful. In addition, low libido can often be explained by chronic disease, such as diabetes, cancer, or clinical depression.
Some medications, including selective serotonin reuptake inhibitors (SSRIs), frequently cause a decline in sexual interest. Finally, psychosocial and partner issues may be the culprit.
James Simon, MD, CCP, NCMP, IF: Much of the beneficial data for testosterone’s use is for sexual function in postmenopausal women.2 Female sexual dysfunction is highly prevalent among women during the postmenopause.3 Androgen levels progressively decrease throughout adult life in all women, so the postmenopausal additional lack of estrogen has a recognized effect on genitourinary health. There is evidence that the insufficiency of androgens as well as estrogens after menopause can lead to genitourinary symptoms of menopause (GSM).4
Testosterone also is used for increasing strength, lean muscle mass, bone mineral density, and sense of well-being.5
Rebecca Glaser, MD: I consider testosterone supplementation in my clinical practice in both premenopausal and postmenopausal women for symptoms of androgen/hormone deficiency, including diminished sense of well-being; dysphoric mood; anxiety; irritability; fatigue; decreased libido, sexual activity, or pleasure; vasomotor instability; bone loss; decreased muscle strength; insomnia; changes in cognition; memory loss; urinary symptoms; incontinence; vaginal atrophy and dryness; and joint and muscular pain. We also have shown through preliminary and short-term data and case studies that testosterone therapy has a potential beneficial effect on migraine headaches, as well as active breast cancers in both premenopausal and postmenopausal women.6-10
Continue to: What is appropriate bloodwork?...
What is appropriate bloodwork?
Dr. Karram: Do you obtain blood work before initiating testosterone treatment? If so, what tests do you order and what testosterone levels are considered to be normal for premenopausal and postmenopausal women?
Dr. Streicher: Unlike estrogen, which is predictably low in a postmenopausal woman, serum testosterone (T) levels are highly variable because of the adrenal component. Ovarian testosterone production does not cease at the same time as estrogen production. So I do obtain total and free T levels, prior to initiating treatment. Having said that, it has been well established that T levels correlate poorly with level of sexual interest, and there is no specific blood level that can be used to differentiate women with and without sexual dysfunction. We all have patients who have nonexistent T levels and have a very healthy libido, and other women with sky-high levels who have no libido. But it is useful to know levels prior to initiating therapy to be able to monitor levels throughout treatment. Also, if levels are in the premenopausal physiologic range, not only is she unlikely to respond but she is also at risk for developing androgenic adverse effects, such as acne and hair growth. In general, a low free T level (even if it is in the normal postmenopausal range) in a clinical setting of HSDD supports supplementation.
The assessment and interpretation of T levels can be challenging, particularly as the majority of testosterone is protein-bound and biologically inactive. Free T levels (the biologically active testosterone) in many labs are unreliable and need to be calculated.
In addition to total and free T, I check levels of sex hormone-binding globulin (SHBG), the protein that binds testosterone and renders it biologically inactive. If someone has high SHBG levels and is taking an oral estrogen, simply switching to a transdermal estrogen will result in decreased SHBG and increased free T.
Levels of total and free T vary from lab to lab, so it is best to be familiar with those ranges and then be consistent in which lab you choose.
Dr. Glaser: Although I personally do order blood work on most patients (T, free T, estradiol, complete blood count, thyroid-stimulating hormone, and follicle-stimulating hormone), after 15 years of research and publishing data on testosterone implants, I do not believe that T levels are absolutely necessary or even beneficial in most cases. It rarely changes management in my patients.
As Lauren said, it is well known that T levels do not correlate with androgen deficiency symptoms or clinical conditions caused by androgen deficiency. If a patient has symptoms of androgen deficiency, a trial of testosterone therapy should be given.
T levels are not a valid marker of tissue exposure in women, reflecting less than 20% of total androgen activity. The major source of testosterone in pre and postmenopausal women is the local intracrine production of testosterone from the adrenal precursor steroids dehydroepiandrosterone (DHEA) and androstenedione, which would not be reflected in T levels.
In our study involving 300 women, we found no relationship between baseline T levels, presenting symptoms, or response to therapy.6 Premenopausal and postmenopausal women had similar baseline T levels and similar response to therapy. Even women with baseline T levels in the mid-range responded to therapy.
Some of the most controversial topics in treating women with testosterone are related to dosing and T levels throughout therapy. Guideline authors often use the terms ‘physiologic dosing’ and ‘physiologic ranges’ when making recommendations for therapy. Although “physiologic” sounds appropriate/ scientific, these rigid opinions/recommendations are not evidence based. There are no data supporting the use of endogenous T ranges to guide dosing or monitor testosterone therapy.
The decision to initiate testosterone therapy is a clinical decision between the doctor and the patient based on the patient’s symptomatology, which is the therapeutic endpoint. Testosterone therapy must be done with adequate doses determined by clinical effect (benefits) versus side effects or adverse events (risks). T levels may be helpful, along with clinical evaluation when troubleshooting.
Utilizing data from thousands of patients, we have developed serum ranges for testosterone implants.11 Even so, no two patients are the same, nor do they respond to therapy the same. It is always a clinical decision.
Continue to: Dr. Simon...
Dr. Simon: In the recent global consensus statement on testosterone use,1 the experts were in agreement that “no cut-off blood level can be used for any measured circulating androgen to differentiate women with and without sexual dysfunction.” They give their recommendation a C, and I agree that testosterone supplementation, with specific dosage levels, are a clinical decision.
Before initiating testosterone therapy, it is recommended that liver function and fasting lipids are assessed, as liver disease and hyperlipidemia are contraindications to treatment. These levels should be monitored twice in the first year and annually thereafter while the patient is taking testosterone. Breast and pelvic examinations, mammography, and evaluation for abnormal bleeding should be performed as well as the blood tests.12 These recommendations are focused on safety not efficacy.
Administration route
Dr. Karram: How do you administer testosterone, and why?
Dr. Streicher: As there are no FDA approved testosterone products for women, clinicians must determine the dosage and route of delivery based on published clinical trials.
Dr. Glaser: I treat patients with subcutaneous pellet implants. The implants provide consistent and continuous delivery of therapeutic amounts of testosterone. There is a reason testosterone pellets have been used for more than 80 years and are more popular now than ever—they work. The insertion procedure is simple and takes about 2 minutes. The treatment is cost-effective, avoids first pass, has no adverse effect on the liver or clotting factors, and there is no transference. Decades of data support both the efficacy and safety of testosterone implants.6 However, testosterone implants are not regulated by the FDA and all patients are required to sign a consent informing them of off-label use, benefits, and risks of testosterone implant therapy.
Dr. Simon: I think the consent is important, as there is no package labeling to warn of possible side effects.
Dr. Streicher: Oral testosterone therapy, because of its first pass through the liver and association with adverse lipid profiles with negative effects on high- and low-density lipoprotein cholesterol levels, is not recommended. I prefer a transdermal approach. Pellets, implants, and injections have the potential to result in supraphysiologic blood concentrations. It must be emphasized that the goal of treatment is to approximate premenopausal physiologic levels. More is not better; excessive levels do not demonstrate a greater sexual response and are in fact more likely to have a negative impact due to androgenic side effects.
In most clinical trials, a 300 mg/d testosterone patch was effective, but these patches are not commercially available so I rely on transdermal gel from a compounding pharmacy. The typical dose needed to raise levels into the high to normal range for most women is 2.5 mg up to 5 mg per day of testosterone 1%, which translates to roughly 1 mL. Many pharmacies provide a dispenser, which allots the appropriate dose. Alternatively, I instruct the patient to place a dollop on her thigh (roughly in size of a single M&M candy).
I always tell my patients that the response is not immediate, typically taking 8 to 12 weeks for the effect to become clinically significant. I generally see a patient back 8 weeks after initiation of treatment to check T levels and evaluate response.
Dr. Simon: There are some data demonstrating that intravaginal testosterone can be a potential treatment for GSM. Intravaginal testosterone coupled with aromatase inhibitor therapy used for breast cancer treatment resulted in supraphysiologic T levels and reportedly improved vaginal maturation index and reduced dyspareunia. More study is needed.13
Dr. Streicher: Agreed. The lower third of the vagina and the vestibule is rich in testosterone receptors. Like Dr. Simon, in some cases of vaginal atrophy I prescribe a compounded local vaginal testosterone.
Continue to: Testosterone and premenopausal women...
Testosterone and premenopausal women
Dr. Karram: Is there a role for testosterone supplementation in premenopausal women with normal estrogen production?
Dr. Glaser: Yes. In fact, in our study, more than one-third of the patients were premenopausal, which makes sense.6 There is a marked decline of T levels and the adrenal precursor steroids (DHEA and androstenedione) in women between the ages of 20–30 years and around age 50. As we said, symptoms of androgen deficiency often occur prior to menopause and are not related to estrogen levels. In our study, testosterone implant therapy relieved symptoms of hormone (androgen) deficiency, including vasomotor symptoms, sleep problems, depressive mood, irritability, anxiety, physical and mental exhaustion (fatigue, memory issues), sexual problems, bladder problems (incontinence, frequency), vaginal dryness, and joint and muscular pain. Premenopausal and postmenopausal patients reported similar hormone deficiency symptoms. Premenopausal women did report a higher incidence of psychological complaints (depressive mood, anxiety, and irritability), while postmenopausal women reported more hot flashes, vaginal dryness, and urologic symptoms. Both groups demonstrated similar improvement in symptoms.
In addition, we have seen relief of severe migraine headache in premenopausal (as well as postmenopausal) women treated with testosterone implant therapy.6,7
Dr. Streicher: The goal of testosterone supplementation is to approximate physiological testosterone concentrations for premenopausal women. While testosterone may improve well-being and sexual function in premenopausal women, the data are limited and really inconclusive. More study is needed given that there is likely a wide therapeutic range with many variables. Having said that, there are some data that indicate that testosterone in premenopausal women may enhance general sense of well-being.14
Why is there no FDA-approved agent?
Dr. Karram: Why do you think the FDA has been reluctant to approve a testosterone agent for women?
Dr. Simon: Three potential testosterone drugs for use in women have been unsuccessfully brought to market after the FDA did not approve them. There are 31 approved products for men, each of which were approved because they safely restored normal testosterone concentrations in men with reduced levels and an associated medical condition. Unlike this scenario for men, for women, the FDA has required products to show clinical effectiveness in trials. For instance, Estratest, a combination estrogen-testosterone product, was in use in the 1960s—approved for women with estrogen-resistant hot flushes, and used in practice for sexual dysfunction. After the FDA implemented its Drug Efficacy Study and Implementation regulation system after 2000, which required safety and efficacy trial(s) before drug approval, the manufacturer removed the drug from market when presented efficacy study data for the added testosterone in the drug were deemed inadequate.15
Dr. Streicher: We have yet another example of the disparity between the FDA approval processes for sexual function drugs for men versus women. Take Intrinsia as another example. It was a 300-mg testosterone patch that underwent clinical trials in women who were post-oophorectomy with HSDD. The patch had demonstrated efficacy with minimal adverse effects and no statistically significant dangerous effects. However, the FDA declined approval, citing “safety considerations” and requested longer-term clinical trials to evaluate potential cardiovascular or breast problems. Given that Intrinsia supplementation simply restored normal physiologic testosterone levels, and there was no such requirement in men who received supplementation post-orchiectomy, this requirement was nonsensical and unjustified.
Compounded formulations
Dr. Karram: Are compounding pharmacies appropriately regulated, and how can you be assured that the source of your testosterone is appropriate?
Dr. Glaser: Compounding pharmacies are regulated by the State Boards of Pharmacy, Drug Enforcement Agency, Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, State Bureaus of Narcotics and Dangerous Drugs, and Departments of Health (in some states).
Compounding is a highly regulated profession that is constantly under scrutiny by agencies, patients, and physicians. Any additional regulations could adversely impact the accessibility of patients to individually compounded medications including intravenous and oncology medications. Over the past 20 years, I have treated hundreds of patients with breast cancer with compounded vaginal testosterone (with or without estriol) and subcutaneous testosterone (with or without anastrozole), greatly improving quality of life in women suffering from severe symptoms. Without the availability of compounded medications, there would have been no or limited alternatives for adequate and much needed therapy. Notably, there have been no adverse events or safety-related issues in more than 20 years.
Regarding whether or not “the source of your testosterone is appropriate,” pharmacists can only use United States Pharmacopeia (USP) grades of testosterone. Testosterone used in compounding is required by the FDA to be of USP grade from an FDA registered and compliant facility. In addition, compounding support companies run additional USP tests to confirm their products meet USP standards prior to being delivered to individual compounding pharmacies.
Dr. Streicher: However, there potentially can be substantial variability between formulations and batches. Product purity can also be an issue. It is reassuring if the compounding pharmacy is compliant with purity of Active Pharmaceutical Ingredients and Good Manufacturing Practice rules and guidelines that assure the minimum requirements to assure high quality and batch-to-batch consistency. I find it helpful to always work with the same pharmacy once you have established uniformity and reliability. If there is concern, it is appropriate to check a patient’s serum level 2 weeks after initiation of therapy.
Dr. Simon: I think the problem with some compounding pharmacies is that there may be incentives back and forth with the clinician to use a certain outlet, whereby the patient’s best interest may not be served. I do believe that there is a role for compounding pharmacies, however. We also use them because some women may have strange reactions or be allergic to the preservatives, formulating agents, or even lactose, in various pills and patches, gels, and creams.
Continue to: Testosterone for aging and cognition?...
Testosterone for aging and cognition?
Dr. Karram: Do you think that testosterone supplementation in the elderly can have a positive impact on aging, Alzheimer disease, and dementia?
Dr. Streicher: The jury is still out on the cognitive effects of postmenopausal androgen supplementation. There is currently insufficient evidence to support the use of testosterone to enhance cognitive performance, or to delay cognitive decline. I prescribe testosterone only to treat HSDD, but I do tell my patient that she may possibly also benefit in terms of cognitive function, musculoskeletal parameters, and well-being. Large RCTs are needed in those areas to justify prescribing for those benefits alone.
Dr. Simon: I would say this is the place for future development, but where there is very likely to be a benefit is on sarcopenia.
Dr. Glaser: There is some evidence that testosterone is neuroprotective.16 In my clinical practice I have seen “self-reported” memory issues improved on therapy, often returning toward the end of the testosterone implant cycle. Adequate amounts of bioavailable testosterone at the androgen receptor are critical for optimal health, immune function, and disease prevention.
Dr. Karram: In conclusion, this expert panel agrees that testosterone supplementation is beneficial for sexual dysfunction in postmenopausal women, with also many other potential benefits that require further investigation. Route of administration preferred by Dr. Simon and Dr. Streicher is transdermal or a transvaginal cream. Dr. Glaser uses a subcutaneous pellet approach. Thank you all for an engaging and informative discussion. ●
Dr. Karram: How would you treat the following patient? She is 56, postmenopausal, and taking estrogen. She reports decreased libido, fatigue, lack of sleep, and lack of focus. Would you consider testosterone supplementation?
Dr. Simon: For her libido, yes. I would not give it to her for the fatigue if it were simply lack of sleep and without an associated medical condition. For her lack of focus, the testosterone could be beneficial. The central nervous system effects of testosterone are thought to be related to the conversion of testosterone to estrogen in the brain; if a person’s getting enough estrogen, they shouldn’t have lack of focus. Since some women may not want more estrogen, administering a little testosterone for libido also offers focus because it adds to the estrogen in the brain. If after giving her adequate amounts of testosterone her libido is not better in 8 weeks, it wasn’t a testosterone problem. If she does report improvement, however, I would keep her on the agent as long as she is healthy. But most 56-year-old women who already met the criteria for going on estrogen should be fine with testosterone.
If this same patient were not reporting low libido but did report lack of strength, energy, or well-being I also would say, “Sure, give testosterone a try.”
Dr. Glaser: I also would treat her with testosterone—with pellet implants. The dose would depend on her body weight. I usually start with an approximate dose of 1 mg of testosterone per pound of body weight. This amount of testosterone delivered continuously from the implant also supplies estradiol (via aromatization) locally at the cellular level.
I would treat her for as long as she chooses to continue testosterone therapy. There is no end- or stop-date where a person no longer benefits from therapy or adverse events occur. Testosterone does not increase the risk of breast cancer and it has a positive effect on many of the adverse signs and symptoms of aging, including mental and physical deterioration.
There are no currently US Food and Drug Administration (FDA)-approved therapies for testosterone use in women. Its use by clinicians is through dose modification of FDA-approved therapies for men, or preparations created by compounding pharmacies. Recently, several professional organizations, including the American College of Obstetricians and Gynecologists (ACOG), North American Menopause Society, International Society for the Study of Women’s Sexual Health, and the International Society for Sexual Medicine, convened an expert panel to develop a global position statement on testosterone therapy for women.1 In this roundtable for OBG Management, moderated by Mickey Karram, MD, several experts discuss this position statement as well as the overall clinical advantages and drawbacks of using testosterone in women.
Testosterone indications
Mickey Karram, MD: For which indications do you prescribe testosterone supplementation in women?
Lauren Streicher, MD: I offer systemic testosterone therapy to postmenopausal women who have hypoactive sexual desire disorder (HSDD) and low serum testosterone levels, with one caveat—it is important that the patient’s reported distressing lack of libido is not explained by another condition or circumstance. Many women present reporting low libido but, on further questioning, it is typically revealed that dyspareunia precipitated their loss of interest in sex. It is normal to not want to do something that is painful. In addition, low libido can often be explained by chronic disease, such as diabetes, cancer, or clinical depression.
Some medications, including selective serotonin reuptake inhibitors (SSRIs), frequently cause a decline in sexual interest. Finally, psychosocial and partner issues may be the culprit.
James Simon, MD, CCP, NCMP, IF: Much of the beneficial data for testosterone’s use is for sexual function in postmenopausal women.2 Female sexual dysfunction is highly prevalent among women during the postmenopause.3 Androgen levels progressively decrease throughout adult life in all women, so the postmenopausal additional lack of estrogen has a recognized effect on genitourinary health. There is evidence that the insufficiency of androgens as well as estrogens after menopause can lead to genitourinary symptoms of menopause (GSM).4
Testosterone also is used for increasing strength, lean muscle mass, bone mineral density, and sense of well-being.5
Rebecca Glaser, MD: I consider testosterone supplementation in my clinical practice in both premenopausal and postmenopausal women for symptoms of androgen/hormone deficiency, including diminished sense of well-being; dysphoric mood; anxiety; irritability; fatigue; decreased libido, sexual activity, or pleasure; vasomotor instability; bone loss; decreased muscle strength; insomnia; changes in cognition; memory loss; urinary symptoms; incontinence; vaginal atrophy and dryness; and joint and muscular pain. We also have shown through preliminary and short-term data and case studies that testosterone therapy has a potential beneficial effect on migraine headaches, as well as active breast cancers in both premenopausal and postmenopausal women.6-10
Continue to: What is appropriate bloodwork?...
What is appropriate bloodwork?
Dr. Karram: Do you obtain blood work before initiating testosterone treatment? If so, what tests do you order and what testosterone levels are considered to be normal for premenopausal and postmenopausal women?
Dr. Streicher: Unlike estrogen, which is predictably low in a postmenopausal woman, serum testosterone (T) levels are highly variable because of the adrenal component. Ovarian testosterone production does not cease at the same time as estrogen production. So I do obtain total and free T levels, prior to initiating treatment. Having said that, it has been well established that T levels correlate poorly with level of sexual interest, and there is no specific blood level that can be used to differentiate women with and without sexual dysfunction. We all have patients who have nonexistent T levels and have a very healthy libido, and other women with sky-high levels who have no libido. But it is useful to know levels prior to initiating therapy to be able to monitor levels throughout treatment. Also, if levels are in the premenopausal physiologic range, not only is she unlikely to respond but she is also at risk for developing androgenic adverse effects, such as acne and hair growth. In general, a low free T level (even if it is in the normal postmenopausal range) in a clinical setting of HSDD supports supplementation.
The assessment and interpretation of T levels can be challenging, particularly as the majority of testosterone is protein-bound and biologically inactive. Free T levels (the biologically active testosterone) in many labs are unreliable and need to be calculated.
In addition to total and free T, I check levels of sex hormone-binding globulin (SHBG), the protein that binds testosterone and renders it biologically inactive. If someone has high SHBG levels and is taking an oral estrogen, simply switching to a transdermal estrogen will result in decreased SHBG and increased free T.
Levels of total and free T vary from lab to lab, so it is best to be familiar with those ranges and then be consistent in which lab you choose.
Dr. Glaser: Although I personally do order blood work on most patients (T, free T, estradiol, complete blood count, thyroid-stimulating hormone, and follicle-stimulating hormone), after 15 years of research and publishing data on testosterone implants, I do not believe that T levels are absolutely necessary or even beneficial in most cases. It rarely changes management in my patients.
As Lauren said, it is well known that T levels do not correlate with androgen deficiency symptoms or clinical conditions caused by androgen deficiency. If a patient has symptoms of androgen deficiency, a trial of testosterone therapy should be given.
T levels are not a valid marker of tissue exposure in women, reflecting less than 20% of total androgen activity. The major source of testosterone in pre and postmenopausal women is the local intracrine production of testosterone from the adrenal precursor steroids dehydroepiandrosterone (DHEA) and androstenedione, which would not be reflected in T levels.
In our study involving 300 women, we found no relationship between baseline T levels, presenting symptoms, or response to therapy.6 Premenopausal and postmenopausal women had similar baseline T levels and similar response to therapy. Even women with baseline T levels in the mid-range responded to therapy.
Some of the most controversial topics in treating women with testosterone are related to dosing and T levels throughout therapy. Guideline authors often use the terms ‘physiologic dosing’ and ‘physiologic ranges’ when making recommendations for therapy. Although “physiologic” sounds appropriate/ scientific, these rigid opinions/recommendations are not evidence based. There are no data supporting the use of endogenous T ranges to guide dosing or monitor testosterone therapy.
The decision to initiate testosterone therapy is a clinical decision between the doctor and the patient based on the patient’s symptomatology, which is the therapeutic endpoint. Testosterone therapy must be done with adequate doses determined by clinical effect (benefits) versus side effects or adverse events (risks). T levels may be helpful, along with clinical evaluation when troubleshooting.
Utilizing data from thousands of patients, we have developed serum ranges for testosterone implants.11 Even so, no two patients are the same, nor do they respond to therapy the same. It is always a clinical decision.
Continue to: Dr. Simon...
Dr. Simon: In the recent global consensus statement on testosterone use,1 the experts were in agreement that “no cut-off blood level can be used for any measured circulating androgen to differentiate women with and without sexual dysfunction.” They give their recommendation a C, and I agree that testosterone supplementation, with specific dosage levels, are a clinical decision.
Before initiating testosterone therapy, it is recommended that liver function and fasting lipids are assessed, as liver disease and hyperlipidemia are contraindications to treatment. These levels should be monitored twice in the first year and annually thereafter while the patient is taking testosterone. Breast and pelvic examinations, mammography, and evaluation for abnormal bleeding should be performed as well as the blood tests.12 These recommendations are focused on safety not efficacy.
Administration route
Dr. Karram: How do you administer testosterone, and why?
Dr. Streicher: As there are no FDA approved testosterone products for women, clinicians must determine the dosage and route of delivery based on published clinical trials.
Dr. Glaser: I treat patients with subcutaneous pellet implants. The implants provide consistent and continuous delivery of therapeutic amounts of testosterone. There is a reason testosterone pellets have been used for more than 80 years and are more popular now than ever—they work. The insertion procedure is simple and takes about 2 minutes. The treatment is cost-effective, avoids first pass, has no adverse effect on the liver or clotting factors, and there is no transference. Decades of data support both the efficacy and safety of testosterone implants.6 However, testosterone implants are not regulated by the FDA and all patients are required to sign a consent informing them of off-label use, benefits, and risks of testosterone implant therapy.
Dr. Simon: I think the consent is important, as there is no package labeling to warn of possible side effects.
Dr. Streicher: Oral testosterone therapy, because of its first pass through the liver and association with adverse lipid profiles with negative effects on high- and low-density lipoprotein cholesterol levels, is not recommended. I prefer a transdermal approach. Pellets, implants, and injections have the potential to result in supraphysiologic blood concentrations. It must be emphasized that the goal of treatment is to approximate premenopausal physiologic levels. More is not better; excessive levels do not demonstrate a greater sexual response and are in fact more likely to have a negative impact due to androgenic side effects.
In most clinical trials, a 300 mg/d testosterone patch was effective, but these patches are not commercially available so I rely on transdermal gel from a compounding pharmacy. The typical dose needed to raise levels into the high to normal range for most women is 2.5 mg up to 5 mg per day of testosterone 1%, which translates to roughly 1 mL. Many pharmacies provide a dispenser, which allots the appropriate dose. Alternatively, I instruct the patient to place a dollop on her thigh (roughly in size of a single M&M candy).
I always tell my patients that the response is not immediate, typically taking 8 to 12 weeks for the effect to become clinically significant. I generally see a patient back 8 weeks after initiation of treatment to check T levels and evaluate response.
Dr. Simon: There are some data demonstrating that intravaginal testosterone can be a potential treatment for GSM. Intravaginal testosterone coupled with aromatase inhibitor therapy used for breast cancer treatment resulted in supraphysiologic T levels and reportedly improved vaginal maturation index and reduced dyspareunia. More study is needed.13
Dr. Streicher: Agreed. The lower third of the vagina and the vestibule is rich in testosterone receptors. Like Dr. Simon, in some cases of vaginal atrophy I prescribe a compounded local vaginal testosterone.
Continue to: Testosterone and premenopausal women...
Testosterone and premenopausal women
Dr. Karram: Is there a role for testosterone supplementation in premenopausal women with normal estrogen production?
Dr. Glaser: Yes. In fact, in our study, more than one-third of the patients were premenopausal, which makes sense.6 There is a marked decline of T levels and the adrenal precursor steroids (DHEA and androstenedione) in women between the ages of 20–30 years and around age 50. As we said, symptoms of androgen deficiency often occur prior to menopause and are not related to estrogen levels. In our study, testosterone implant therapy relieved symptoms of hormone (androgen) deficiency, including vasomotor symptoms, sleep problems, depressive mood, irritability, anxiety, physical and mental exhaustion (fatigue, memory issues), sexual problems, bladder problems (incontinence, frequency), vaginal dryness, and joint and muscular pain. Premenopausal and postmenopausal patients reported similar hormone deficiency symptoms. Premenopausal women did report a higher incidence of psychological complaints (depressive mood, anxiety, and irritability), while postmenopausal women reported more hot flashes, vaginal dryness, and urologic symptoms. Both groups demonstrated similar improvement in symptoms.
In addition, we have seen relief of severe migraine headache in premenopausal (as well as postmenopausal) women treated with testosterone implant therapy.6,7
Dr. Streicher: The goal of testosterone supplementation is to approximate physiological testosterone concentrations for premenopausal women. While testosterone may improve well-being and sexual function in premenopausal women, the data are limited and really inconclusive. More study is needed given that there is likely a wide therapeutic range with many variables. Having said that, there are some data that indicate that testosterone in premenopausal women may enhance general sense of well-being.14
Why is there no FDA-approved agent?
Dr. Karram: Why do you think the FDA has been reluctant to approve a testosterone agent for women?
Dr. Simon: Three potential testosterone drugs for use in women have been unsuccessfully brought to market after the FDA did not approve them. There are 31 approved products for men, each of which were approved because they safely restored normal testosterone concentrations in men with reduced levels and an associated medical condition. Unlike this scenario for men, for women, the FDA has required products to show clinical effectiveness in trials. For instance, Estratest, a combination estrogen-testosterone product, was in use in the 1960s—approved for women with estrogen-resistant hot flushes, and used in practice for sexual dysfunction. After the FDA implemented its Drug Efficacy Study and Implementation regulation system after 2000, which required safety and efficacy trial(s) before drug approval, the manufacturer removed the drug from market when presented efficacy study data for the added testosterone in the drug were deemed inadequate.15
Dr. Streicher: We have yet another example of the disparity between the FDA approval processes for sexual function drugs for men versus women. Take Intrinsia as another example. It was a 300-mg testosterone patch that underwent clinical trials in women who were post-oophorectomy with HSDD. The patch had demonstrated efficacy with minimal adverse effects and no statistically significant dangerous effects. However, the FDA declined approval, citing “safety considerations” and requested longer-term clinical trials to evaluate potential cardiovascular or breast problems. Given that Intrinsia supplementation simply restored normal physiologic testosterone levels, and there was no such requirement in men who received supplementation post-orchiectomy, this requirement was nonsensical and unjustified.
Compounded formulations
Dr. Karram: Are compounding pharmacies appropriately regulated, and how can you be assured that the source of your testosterone is appropriate?
Dr. Glaser: Compounding pharmacies are regulated by the State Boards of Pharmacy, Drug Enforcement Agency, Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, State Bureaus of Narcotics and Dangerous Drugs, and Departments of Health (in some states).
Compounding is a highly regulated profession that is constantly under scrutiny by agencies, patients, and physicians. Any additional regulations could adversely impact the accessibility of patients to individually compounded medications including intravenous and oncology medications. Over the past 20 years, I have treated hundreds of patients with breast cancer with compounded vaginal testosterone (with or without estriol) and subcutaneous testosterone (with or without anastrozole), greatly improving quality of life in women suffering from severe symptoms. Without the availability of compounded medications, there would have been no or limited alternatives for adequate and much needed therapy. Notably, there have been no adverse events or safety-related issues in more than 20 years.
Regarding whether or not “the source of your testosterone is appropriate,” pharmacists can only use United States Pharmacopeia (USP) grades of testosterone. Testosterone used in compounding is required by the FDA to be of USP grade from an FDA registered and compliant facility. In addition, compounding support companies run additional USP tests to confirm their products meet USP standards prior to being delivered to individual compounding pharmacies.
Dr. Streicher: However, there potentially can be substantial variability between formulations and batches. Product purity can also be an issue. It is reassuring if the compounding pharmacy is compliant with purity of Active Pharmaceutical Ingredients and Good Manufacturing Practice rules and guidelines that assure the minimum requirements to assure high quality and batch-to-batch consistency. I find it helpful to always work with the same pharmacy once you have established uniformity and reliability. If there is concern, it is appropriate to check a patient’s serum level 2 weeks after initiation of therapy.
Dr. Simon: I think the problem with some compounding pharmacies is that there may be incentives back and forth with the clinician to use a certain outlet, whereby the patient’s best interest may not be served. I do believe that there is a role for compounding pharmacies, however. We also use them because some women may have strange reactions or be allergic to the preservatives, formulating agents, or even lactose, in various pills and patches, gels, and creams.
Continue to: Testosterone for aging and cognition?...
Testosterone for aging and cognition?
Dr. Karram: Do you think that testosterone supplementation in the elderly can have a positive impact on aging, Alzheimer disease, and dementia?
Dr. Streicher: The jury is still out on the cognitive effects of postmenopausal androgen supplementation. There is currently insufficient evidence to support the use of testosterone to enhance cognitive performance, or to delay cognitive decline. I prescribe testosterone only to treat HSDD, but I do tell my patient that she may possibly also benefit in terms of cognitive function, musculoskeletal parameters, and well-being. Large RCTs are needed in those areas to justify prescribing for those benefits alone.
Dr. Simon: I would say this is the place for future development, but where there is very likely to be a benefit is on sarcopenia.
Dr. Glaser: There is some evidence that testosterone is neuroprotective.16 In my clinical practice I have seen “self-reported” memory issues improved on therapy, often returning toward the end of the testosterone implant cycle. Adequate amounts of bioavailable testosterone at the androgen receptor are critical for optimal health, immune function, and disease prevention.
Dr. Karram: In conclusion, this expert panel agrees that testosterone supplementation is beneficial for sexual dysfunction in postmenopausal women, with also many other potential benefits that require further investigation. Route of administration preferred by Dr. Simon and Dr. Streicher is transdermal or a transvaginal cream. Dr. Glaser uses a subcutaneous pellet approach. Thank you all for an engaging and informative discussion. ●
Dr. Karram: How would you treat the following patient? She is 56, postmenopausal, and taking estrogen. She reports decreased libido, fatigue, lack of sleep, and lack of focus. Would you consider testosterone supplementation?
Dr. Simon: For her libido, yes. I would not give it to her for the fatigue if it were simply lack of sleep and without an associated medical condition. For her lack of focus, the testosterone could be beneficial. The central nervous system effects of testosterone are thought to be related to the conversion of testosterone to estrogen in the brain; if a person’s getting enough estrogen, they shouldn’t have lack of focus. Since some women may not want more estrogen, administering a little testosterone for libido also offers focus because it adds to the estrogen in the brain. If after giving her adequate amounts of testosterone her libido is not better in 8 weeks, it wasn’t a testosterone problem. If she does report improvement, however, I would keep her on the agent as long as she is healthy. But most 56-year-old women who already met the criteria for going on estrogen should be fine with testosterone.
If this same patient were not reporting low libido but did report lack of strength, energy, or well-being I also would say, “Sure, give testosterone a try.”
Dr. Glaser: I also would treat her with testosterone—with pellet implants. The dose would depend on her body weight. I usually start with an approximate dose of 1 mg of testosterone per pound of body weight. This amount of testosterone delivered continuously from the implant also supplies estradiol (via aromatization) locally at the cellular level.
I would treat her for as long as she chooses to continue testosterone therapy. There is no end- or stop-date where a person no longer benefits from therapy or adverse events occur. Testosterone does not increase the risk of breast cancer and it has a positive effect on many of the adverse signs and symptoms of aging, including mental and physical deterioration.
- Davis SR, Baber R, Panay N, et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. Climacteric. 2019;22:429-434.
- Islam RM, Bell RJ, Green S, et al. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Diabetes Endocrinol. 2019;S2213-8587:30189-30195.
- Simon JA, Davis SR, Althof SE, et al. Sexual well-being after menopause: an International Menopause Society White Paper. Climacteric. 2018;21:415-427.
- Traish AM, Vignozzi L, Simon JA, et al. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev. 2018;6:558-571.
- Panay N. British Menopause Society tools for clinicians: testosterone replacement in menopause. Post Reprod Health. 2019;25:40-42.
- Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas. 2011;68:355-361.
- Glaser R, Dimitrakakis C, Trimble N, et al. Testosterone pellet implants and migraine headaches: a pilot study. Maturitas. 2012;71:385-388.
- Glaser RL, York AE, Dimitrakakis C. Efficacy of subcutaneous testosterone on menopausal symptoms in breast cancer survivors. J Clin Oncol. 2014;32(suppl):109-109.
- Glaser RL, Dimitrakakis C. Rapid response of breast cancer to neoadjuvant intramammary testosterone-anastrozole therapy: neoadjuvant hormone therapy in breast cancer. Menopause. 2014;21:673.
- Glaser RL, York AE, Dimitrakakis C. Subcutaneous testosterone-letrozole therapy before and concurrent with neoadjuvant breast chemotherapy: clinical response and therapeutic implications. Menopause. 2017;24:859-864.
- Glaser R, Kalantaridou S, Dimitrakakis C, et al. Testosterone implants in women: pharmacological dosing for a physiologic effect. Maturitas. 2013;74:179-184.
- International Society for the Study of Women’s Sexual Health (ISSWSH) clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. In press.
- Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause. 2018;25:837-847.
- Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
- Simon JA, Kapner MD. The saga of testosterone for menopausal women at the Food and Drug Administration (FDA). J Sex Med. 2020;17:826-829.
- Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3: 980-992.
- Davis SR, Baber R, Panay N, et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. Climacteric. 2019;22:429-434.
- Islam RM, Bell RJ, Green S, et al. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Diabetes Endocrinol. 2019;S2213-8587:30189-30195.
- Simon JA, Davis SR, Althof SE, et al. Sexual well-being after menopause: an International Menopause Society White Paper. Climacteric. 2018;21:415-427.
- Traish AM, Vignozzi L, Simon JA, et al. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev. 2018;6:558-571.
- Panay N. British Menopause Society tools for clinicians: testosterone replacement in menopause. Post Reprod Health. 2019;25:40-42.
- Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas. 2011;68:355-361.
- Glaser R, Dimitrakakis C, Trimble N, et al. Testosterone pellet implants and migraine headaches: a pilot study. Maturitas. 2012;71:385-388.
- Glaser RL, York AE, Dimitrakakis C. Efficacy of subcutaneous testosterone on menopausal symptoms in breast cancer survivors. J Clin Oncol. 2014;32(suppl):109-109.
- Glaser RL, Dimitrakakis C. Rapid response of breast cancer to neoadjuvant intramammary testosterone-anastrozole therapy: neoadjuvant hormone therapy in breast cancer. Menopause. 2014;21:673.
- Glaser RL, York AE, Dimitrakakis C. Subcutaneous testosterone-letrozole therapy before and concurrent with neoadjuvant breast chemotherapy: clinical response and therapeutic implications. Menopause. 2017;24:859-864.
- Glaser R, Kalantaridou S, Dimitrakakis C, et al. Testosterone implants in women: pharmacological dosing for a physiologic effect. Maturitas. 2013;74:179-184.
- International Society for the Study of Women’s Sexual Health (ISSWSH) clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. In press.
- Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause. 2018;25:837-847.
- Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
- Simon JA, Kapner MD. The saga of testosterone for menopausal women at the Food and Drug Administration (FDA). J Sex Med. 2020;17:826-829.
- Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3: 980-992.
Urethral bulking agents for SUI: Rethinking their indications
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
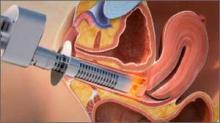
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.
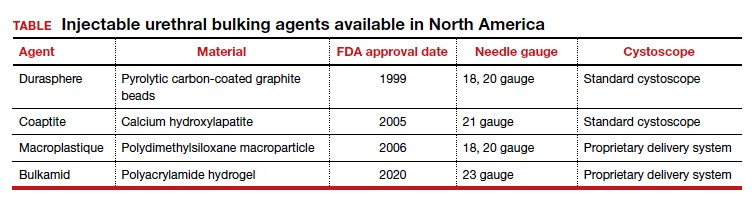
Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4
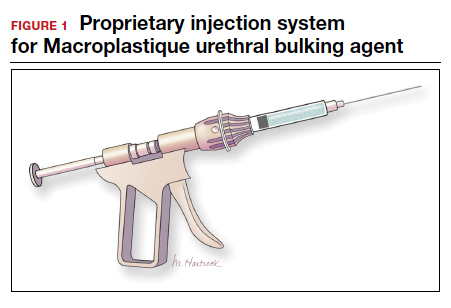

After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).
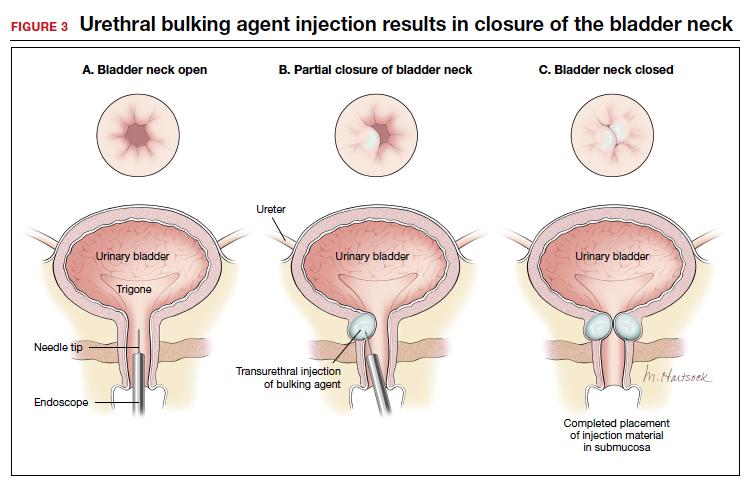
Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Telemedicine: Navigating legal issues
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
Telemedicine: Common hurdles and proper coding for ObGyns
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
Telemedicine: A primer for today’s ObGyn
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.
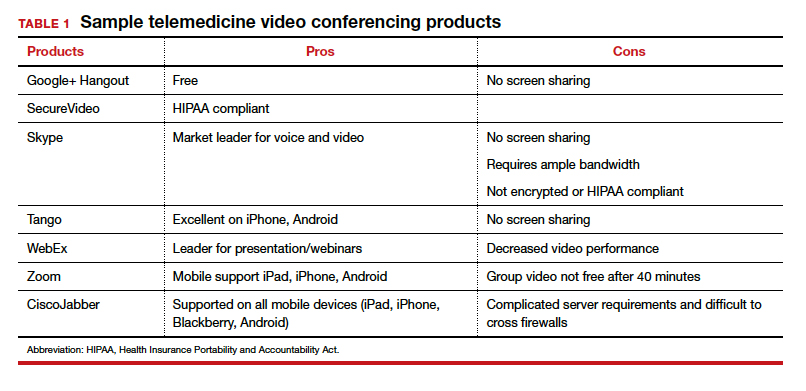
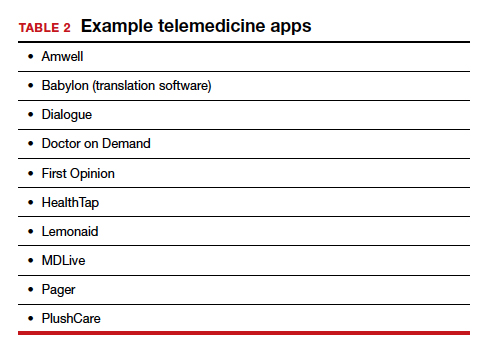
Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
Genitourinary syndrome of menopause: Current and emerging therapies
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 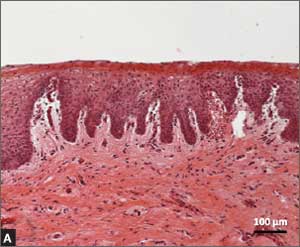 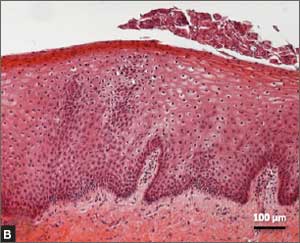 |
FIGURE 2: Atrophic vaginitis 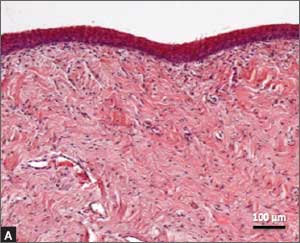 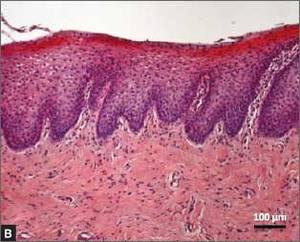 This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
In this article
- What can we offer our patients?
- Fractional CO2 laser: a study in progress
- Bottom line
Urodynamic testing: Who needs it, and key pointers for a successful outcome
More than 300 attendees heard Dr. Mickey Karram address urodynamics and cystoscopy at the annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) in Las Vegas, December 12-14, 2013. Here, a key topic from his presentation.
Dr. Karram is Professor of OB/GYN and Urology, University of Cincinnati School of Medicine, and Director, Urogynecology, The Christ Hospital, Cincinnati, Ohio. He also is Course Director of the Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) and the Female Urology and Urogynecology Symposium (FUUS), both co-sponsored by OBG Management.
FUUS 2014: June 14-16, Aria, Las Vegas
Click here for more info.
More than 300 attendees heard Dr. Mickey Karram address urodynamics and cystoscopy at the annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) in Las Vegas, December 12-14, 2013. Here, a key topic from his presentation.
Dr. Karram is Professor of OB/GYN and Urology, University of Cincinnati School of Medicine, and Director, Urogynecology, The Christ Hospital, Cincinnati, Ohio. He also is Course Director of the Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) and the Female Urology and Urogynecology Symposium (FUUS), both co-sponsored by OBG Management.
FUUS 2014: June 14-16, Aria, Las Vegas
Click here for more info.
More than 300 attendees heard Dr. Mickey Karram address urodynamics and cystoscopy at the annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) in Las Vegas, December 12-14, 2013. Here, a key topic from his presentation.
Dr. Karram is Professor of OB/GYN and Urology, University of Cincinnati School of Medicine, and Director, Urogynecology, The Christ Hospital, Cincinnati, Ohio. He also is Course Director of the Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS) and the Female Urology and Urogynecology Symposium (FUUS), both co-sponsored by OBG Management.
FUUS 2014: June 14-16, Aria, Las Vegas
Click here for more info.
Rectus fascia pubovaginal sling after an unsuccessful TVT
To read the related article, see When and how to place an autologous rectus fascia pubovaginal sling
To read the related article, see When and how to place an autologous rectus fascia pubovaginal sling
To read the related article, see When and how to place an autologous rectus fascia pubovaginal sling
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.
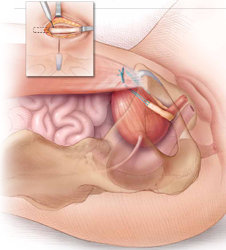
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).
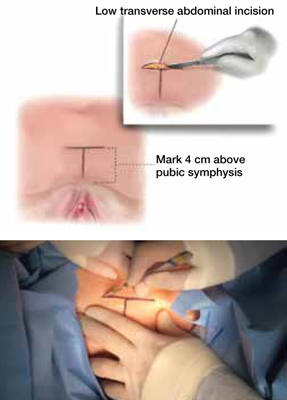
FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).
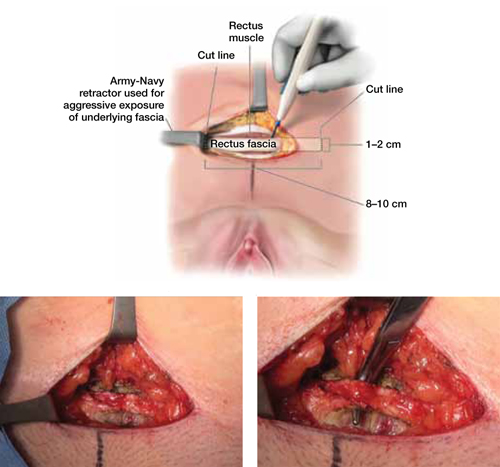
FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).
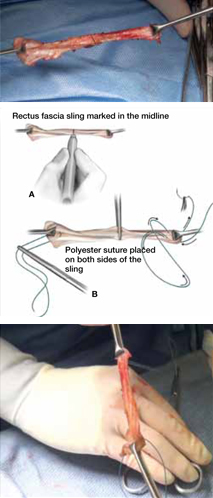
FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).
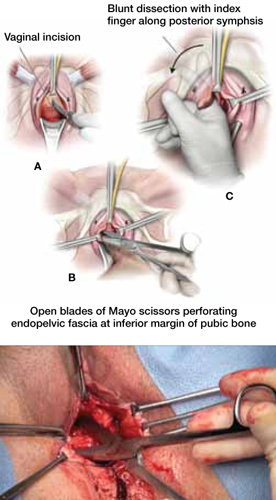
FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.
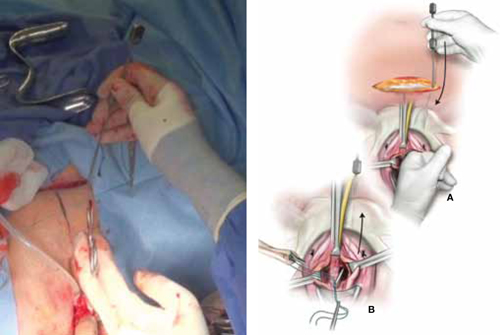
FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).
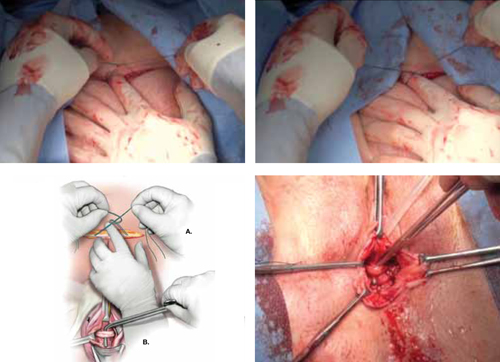
FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.