User login
Fostering surgical innovation: The path forward

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.
Genitourinary syndrome of menopause: Current and emerging therapies
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 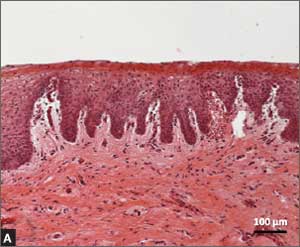 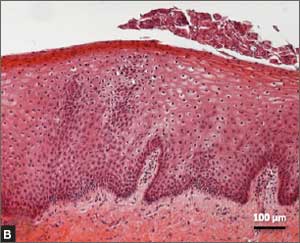 |
FIGURE 2: Atrophic vaginitis 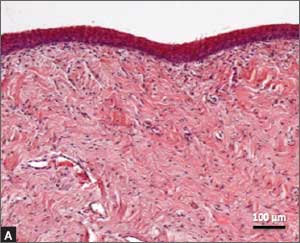 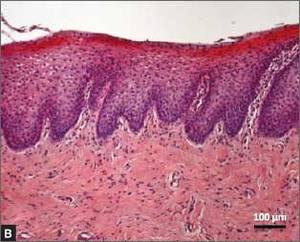 This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
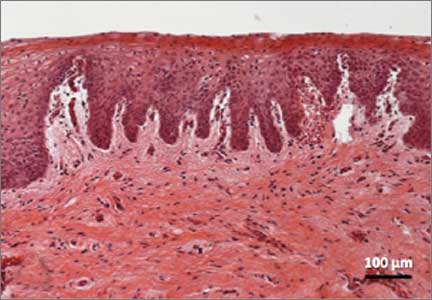
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy   |
FIGURE 2: Atrophic vaginitis   This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
In this article
- What can we offer our patients?
- Fractional CO2 laser: a study in progress
- Bottom line
Expert tips on retropubic vs. transobturator sling approaches
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.
Minimally invasive synthetic midurethral slings may be considered the standard of care for the surgical treatment of stress urinary incontinence – and a first-line treatment for severe cases of the condition – based on the publication of numerous level 1 randomized trials, high-quality reviews, and recent position statements from professional societies.
The current evidence base shows that midurethral sling operations are as effective as bladder neck slings and colposuspension, with less morbidity. Operating times are shorter, and local anesthesia is possible. Compared with pubovaginal slings, which are fixed at the bladder neck, midurethral slings are associated with less postoperative voiding dysfunction and fewer de novo urgency symptoms.
Midurethral slings (MUS) also have been shown to be more successful – and more cost-effective – than pelvic floor physiotherapy for stress urinary incontinence (SUI) overall, with the possible exception of mild SUI.
Physiotherapy involving pelvic floor muscle therapy has long been advocated as a first-line treatment for SUI, with MUS surgery often recommended when physiotherapy is unsuccessful. In recent years, however, with high success rates for MUS, the role of physiotherapy as a first-line treatment has become more debatable.
A multicenter randomized trial in 660 women published last year in the New England Journal of Medicine substantiated what many of us have seen in our practices and in other published studies: significantly lower rates of improvement and cure with initial physiotherapy than with primary surgery.
Initial MUS surgery resulted in higher rates of subjective improvement, compared with initial physiotherapy (91% vs. 64%), subjective cure (85% v. 53%), and objective cure (77% v. 59%) at 1 year. Moreover, a significant number of women – 49% – chose to abandon conservative therapy and have MUS surgery for their SUI during the study period (N. Engl. J. Med. 2013;369:1124-33).
A joint position statement published in early 2014 by the American Urogynecologic Society (AUGS) and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) calls MUS the most extensively studied anti-incontinence procedure and “probably the most important advancement in the treatment of SUI in the last 50 years.” More than 2,000 publications in the literature have described the procedure for SUI, and multiple randomized controlled trials have compared various types of MUS procedures as well as MUS to other nonmesh SUI procedures, the statement says.
My colleague and I recently modeled the cost-effectiveness of pelvic floor muscle therapy and continence pessaries vs. surgical treatment with MUS for initial treatment of SUI. Initial treatment with MUS was the best strategy, with an incremental cost-effectiveness ratio of $32,132 per quality-adjusted life-year, compared with initial treatment with pelvic floor muscle therapy. Under our model, treatment with a continence pessary would never be the preferred choice due to low subjective cure rates (Am. J. Obstet. Gynecol. 2014;211:565.e1-6).
I now tell patients who present with a history of severe stress incontinence, and who leak on a cough stress test, that a trial of pelvic floor physiotherapy is an option but one with a lower likelihood of success. I recommend an MUS as primary treatment for these patients, and the question then often becomes which sling to use.
Sling selection
There are two broad approaches to MUS surgery – retropubic and transobturator – and within each approach, there are different routes for the delivery of the polypropylene mesh sling.
Retropubic slings. Retropubic slings are passed transvaginally at the midurethral level through the retropubic space. Tension-free vaginal tape (TVT) has been used in millions of women worldwide, with good long-term outcomes, since it was introduced by Dr. Ulf Ulmsten in 1995. The TVT procedure utilizes a bottom-up approach, with curved needles being passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
A second type of retropubic sling – the suprapubic urethral support sling (SPARC, American Medical Systems) – utilizes a downward-pass, or top-down, approach in which a metal trocar is passed through suprapubic incisions and down through the retropubic space to exit a vaginal incision.
The theoretical advantages of this modification to the TVT procedure have included more control over the needle introducer near the rectus fascia, and a lower risk of bowel and vascular injury. However, comparisons during the last decade of the two retropubic approaches have suggested slightly better outcomes – relating both to cure rates and to complication rates – with TVT compared with SPARC.
A Cochrane Review published in 2009, titled “Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women,” provided higher-level evidence in favor of bottom-up slings. A sub-meta-analysis of five randomized controlled trials – part of a broader intervention review – showed that a retropubic bottom-up approach was more effective than a top-down route (risk ratio, 1.10), with higher subjective and objective SUI cure rates (Cochrane Database Syst. Rev. 2009(4): CD006375). There also was significantly less bladder perforation, less mesh erosion, and less voiding dysfunction.
TVT slings, therefore, appear to be somewhat superior, with statistically significant differences in each of the domains of efficacy and morbidity. Still, surgeon experience and skill remain factors in sling selection; the surgeon who feels comfortable and skilled with a top-down approach and has little experience with a bottom-up approach should continue with SPARC. For surgeons who are skilled with both approaches, it might well be preferable to favor TVT.
Transobturator slings. The transobturator approach was developed to minimize the potential for bladder and bowel injuries by avoiding the pelvic organs in the retropubic space. The sling is introduced either through an inside-out technique, with the needle passed from a vaginal incision and out through the obturator foramen, or through an outside-in technique, with the needle passed through the thigh and then out through the vaginal incision.
A meta-analysis of trials of transobturator sling procedures – including four direct-comparison, randomized controlled trials of the inside-out technique vs. the outside-in technique – showed no significant differences between the two approaches in subjective and objective SUI cure rates in the short term. Rates of postoperative voiding difficulties and de novo urgency symptoms were similar (BJU Int. 2010;106:68-76).
Making a choice. Each of the currently available midurethral slings appears to work well, overall, with few clinically significant differences in outcomes. On the other hand, midurethral slings are not all the same. It is important to appreciate the more subtle differences, to be aware of the evidence, and to be appropriately trained. Often, sling selection involves weighing the risks and benefits for the individual.
On a broad scale, the most recent high-level comparison of the retropubic and transobturator slings appears to be a meta-analysis in which retropubic midurethral slings showed better objective and subjective cure rates than transobturator midurethral slings. Women treated with retropubic slings had a 35% higher odds of objective cure and a 24% higher odds of subjective cure. (The weighted average objective cure rates were 87% for retropubic slings vs. 83% for transobturator slings with a weighted average follow-up of approximately 17 months. The weighted average subjective cure rates were 76% and 73%, respectively.)
Operating times were longer with retropubic slings, but lengths of stay were equivalent between the two types of procedures. This was based on 17 studies of about 3,000 women (J. Urology 2014 [doi: 10.1016/j.juro.2014.09.104]).
The types of complications seen with each approach differed. Bladder perforation was significantly more common with retropubic slings (3.2% vs. 0.2%), as was bleeding (3.2% v. 1.1%). Transobturator slings were associated with more cases of neurologic symptoms (9.4% v. 3.5%) and vaginal perforation (3.6% v. 0.9%).
This new review provides updated information to the 2009 Cochrane Review mentioned above, which reported that women were less likely to be continent after operations performed via the obturator route, but also less likely to have encountered complications. More specifically, objective cure rates were slightly higher with retropubic slings than with transobturator slings (88% vs. 84%) in the 2009 review. There was no difference in subjective cure rates. With the obturator route, there was less voiding dysfunction, blood loss, and bladder perforation (0.3% v. 5.5%).
Other pivotal trials since the 2009 Cochrane Review include a multicenter randomized equivalence trial published in the New England Journal of Medicine in 2010. The trial randomized 597 women to transobturator or retropubic sling surgery, and found no significant differences in subjective success (56% vs. 62%) or in objective success (78% vs. 81%) at 12 months (N. Engl. J. Med. 2010;362:2066-76).
There is some level 1 evidence suggesting that for severe incontinence involving intrinsic sphincter deficiency (ISD), a retropubic TVT sling is the more effective procedure. A randomized trial of 164 women with urodynamic SUI and ISD, for instance, found that 21% of those in the TVT group and 45% of those in the transobturator group had urodynamic SUI 6 months postoperatively.
The risk ratio of repeat surgery was 2.6 times higher in the transobturator group than in the retropubic TVT group (Obstet. Gynecol. 2008;112:1253-61). TVT was more effective both with and without concurrent pelvic organ prolapse repair.
I tell my patients with severe SUI or ISD, therefore, that retropubic sling procedures appear to be preferable. (Exceptions include the patient who has a history of retropubic surgeries, in whom passing the sling through this route may not be the safest approach, as well as the patient who has had mesh erosion into the bladder.)
In patients whose SUI is less severe, I counsel that a transobturator sling confers satisfaction rates similar to those of a retropubic sling and has a lower risk of complications, such as postoperative voiding dysfunction and bladder perforations, but with the possible trade-off of more thigh discomfort. I also might recommend a transobturator sling to patients with more pronounced initial complaints of urinary urgency and frequency, and to patients who have minor voiding dysfunction or a low level of incomplete bladder emptying.
While often short-lived, the small risk of thigh pain with a transobturator sling makes me less likely to recommend this type of sling for a woman who is a marathon runner or competitive athlete. In her case, an analysis of possible complications includes the consideration that bladder perforation can be addressed relatively quickly in the operating room, while persistent thigh discomfort, though relatively rare, could be a debilitating problem.
Single-incision slings
There appears to be emerging evidence suggesting that some of the fixed and adjustable single-incision slings currently available may have efficacy similar to that of the slings that are now widely used.
A Cochrane Review presented at the 2014 AUGS-IUGA scientific meeting and published this summer concludes that there is not enough evidence on single-incision slings compared with retropubic or transobturator slings to allow reliable comparisons, and that additional, adequately powered, high-quality trials with longer-term follow-up are needed (Cochrane Database Sys. Rev. 2014;6:CD008709). However, research completed since the review offers additional data.
For instance, at the 2014 AUGS-IUGA scientific meeting this summer, an oral paper presentation highlighted findings of a randomized controlled trial that showed similar cure rates after surgery with the MiniArc, a fixed single-incision sling, and the Monarc transobturator sling (both by American Medical Systems) at 24 months. The study randomized 234 women to either sling and found no significant differences in subjective outcomes, objective outcomes, or results on various quality-of-life questionnaires.
As such studies are published and more evidence emerges, we will gain a clearer picture of how the newer single-incision slings compare to the well-tested retropubic and transobturator slings with respect to efficacy and safety.
Single-incision slings require only a small vaginal incision and no exit points. Without abdominal or thigh incisions, these new procedures – intended for less severe SUI (no ISD) – may offer improved perioperative and postoperative patient comfort and a potentially decreased risk of surgical injury to the adductor muscles, as well as a decreased risk of vascular and nerve injury. Candidates for these slings may include those who are very athletic, those who are obese, and those with a history of prior retropubic or pelvic surgery.
Research appears to be progressing, but at this time we do not yet have level 1 evidence to support their routine use.
Dr. Sokol reported that he owns stock in Pelvalon, and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems, and the recipient of research grants from Acell and several other companies.