User login
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.
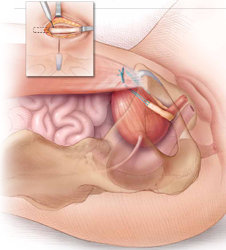
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).
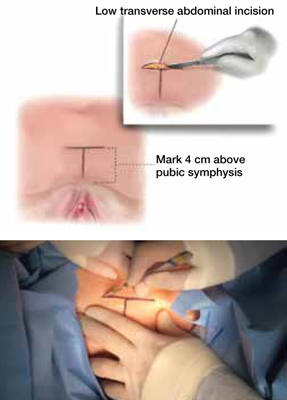
FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).
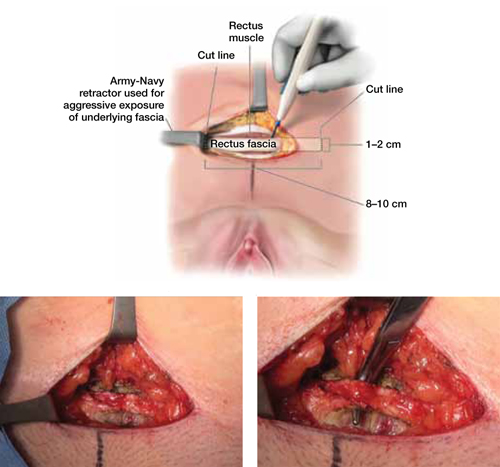
FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).
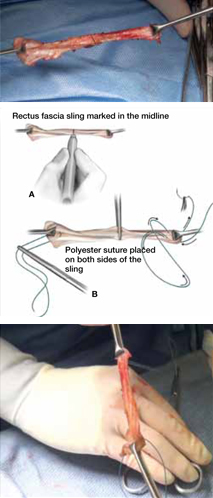
FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).
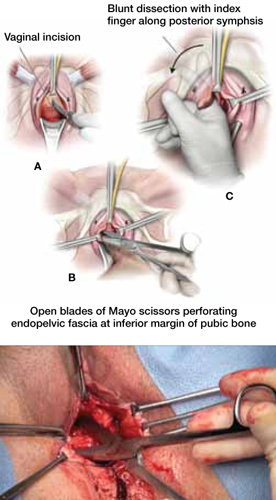
FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.
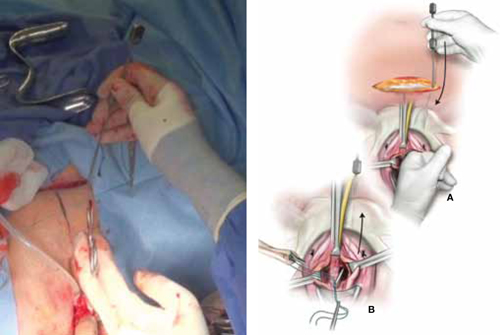
FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).
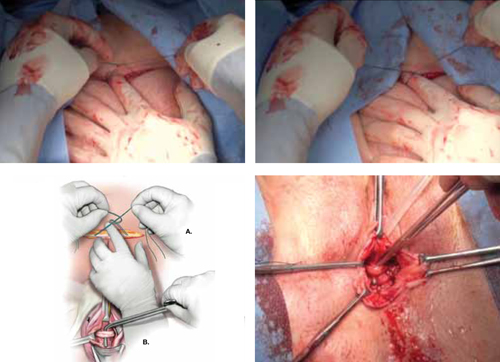
FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.

