User login
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
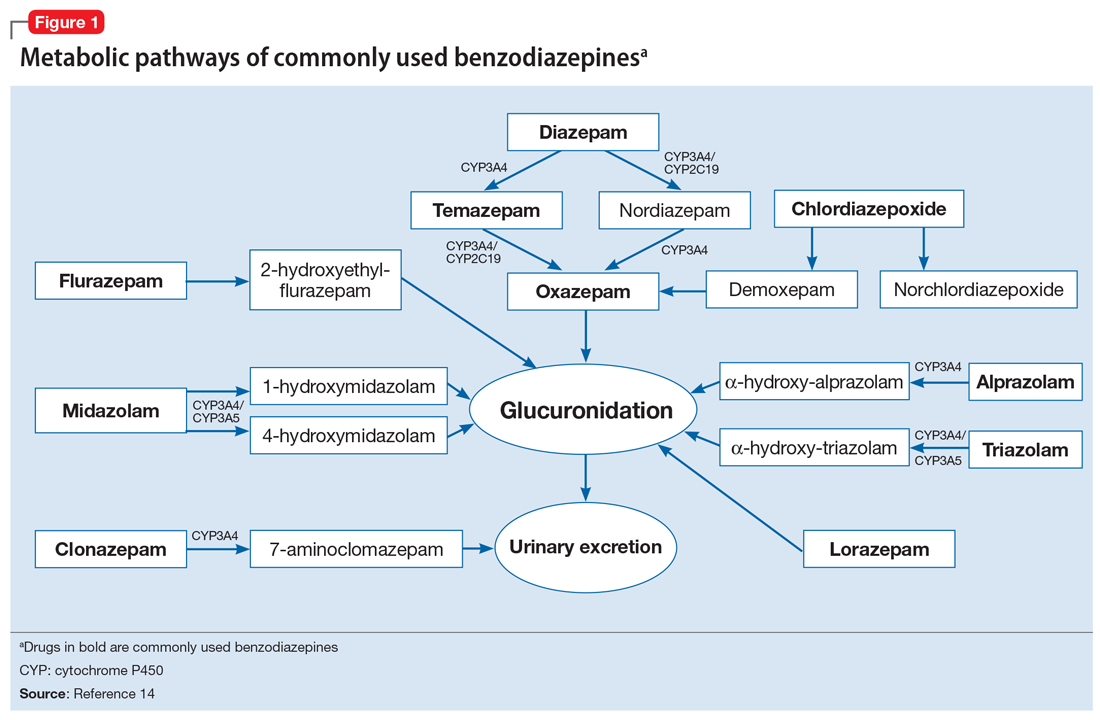
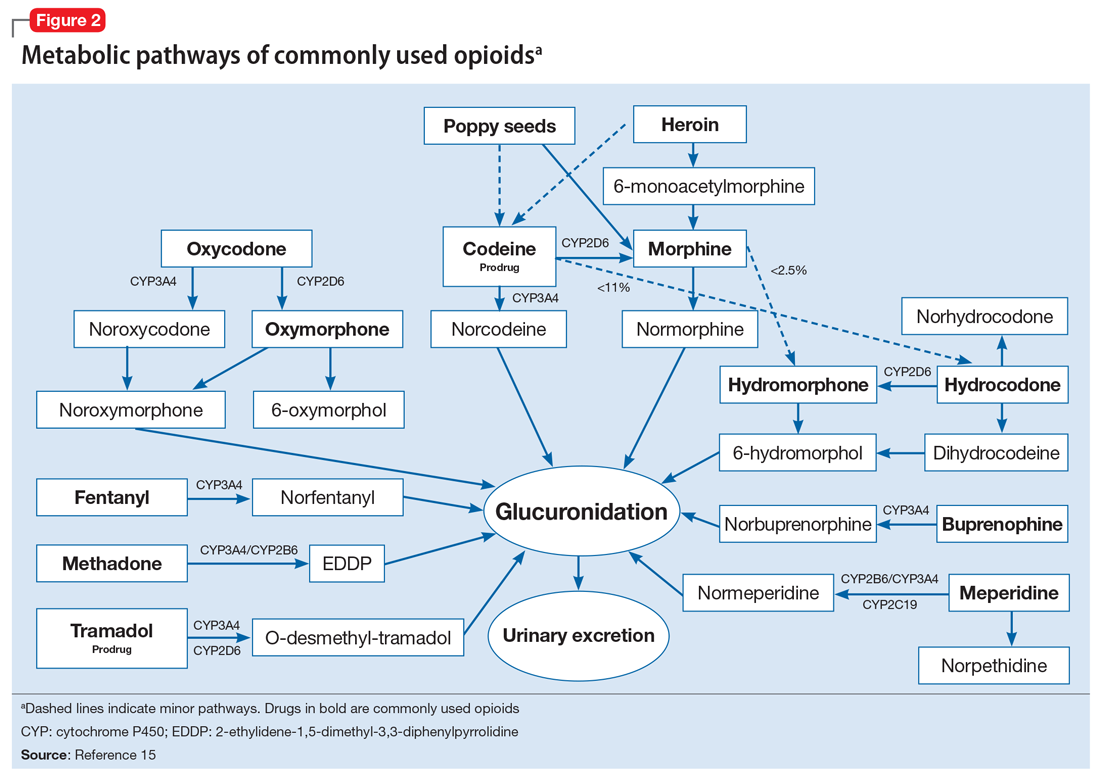
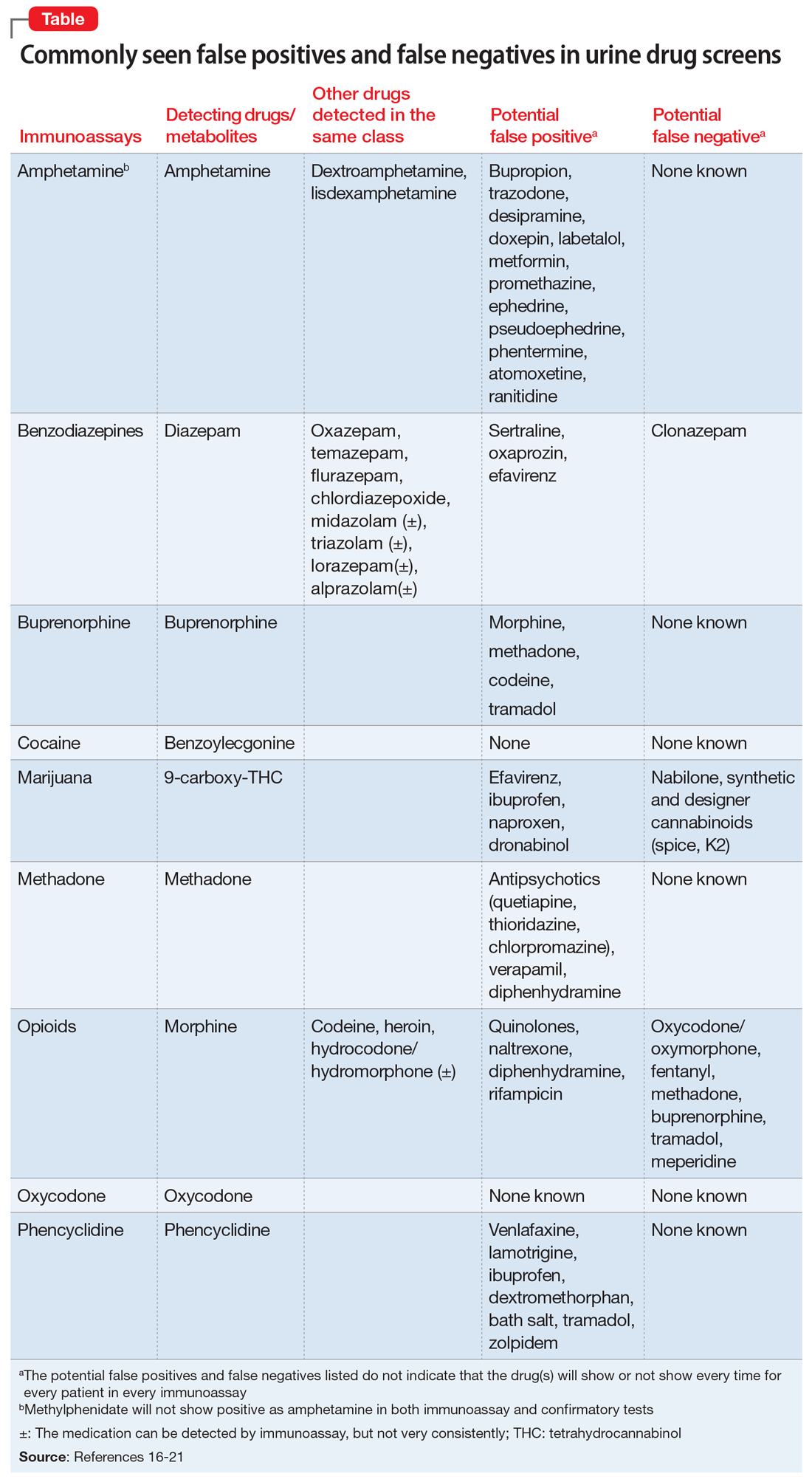
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.
Interpreting UDT results and management strategies
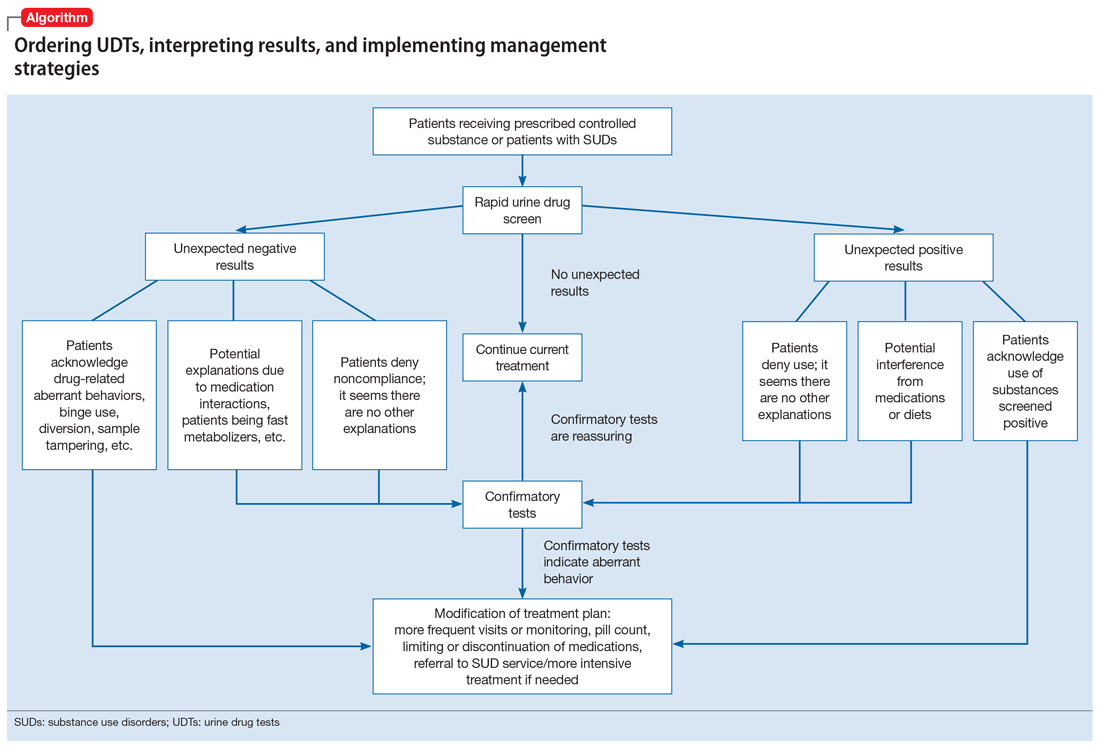
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.
Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.
Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).
Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.
Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.
Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.
Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).
Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.
Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.
Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.
Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).
Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.