Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
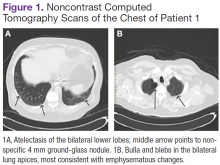
The patient underwent computed tomograpgy (CT) scans of the chest without contrast, which revealed atelectasis, ground-glass opacities, and emphysematous changes (Figures 1A and 1B).The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
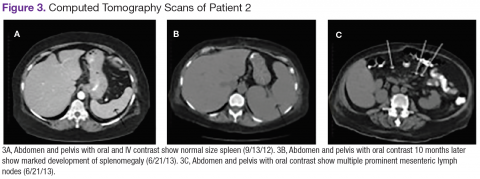
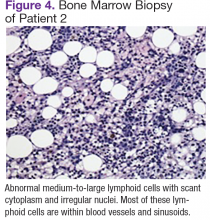
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
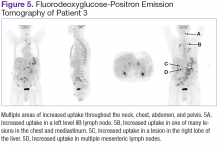
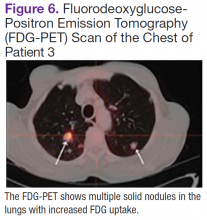
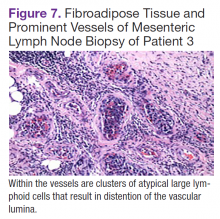
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden. 1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis. 3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis. 11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality. 6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
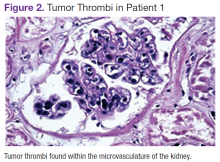
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain. 7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL. 7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis. 1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency. 26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.