User login
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
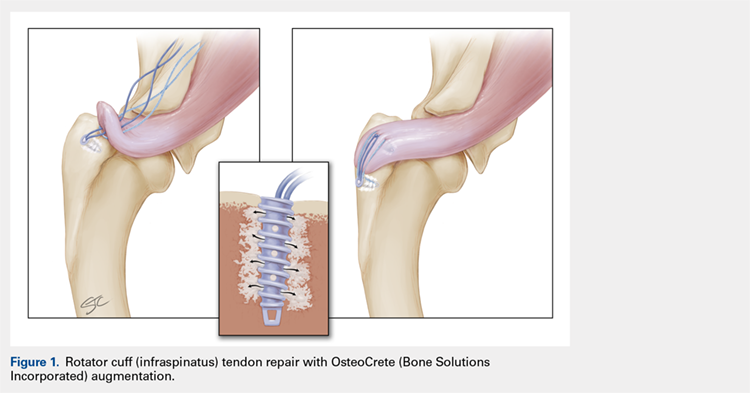
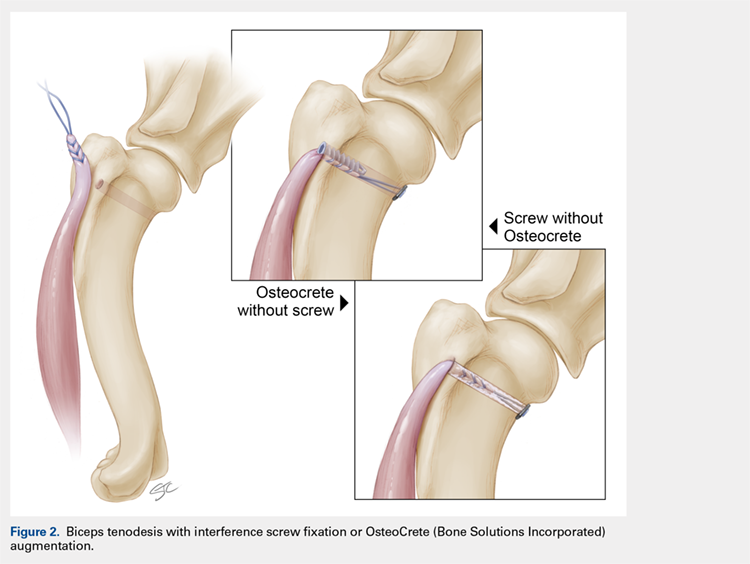
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.
The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20
For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.

The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20

For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.

The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20

For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
TAKE-HOME POINTS
- OsteoCrete, a magnesium-based resorbable bone cement, has potential to safely and effectively augment suture anchor fixation.
- OsteoCrete increases anchor pull-out strength within 15 minutes of injection.
- OsteoCrete has a more profound impact on anchors when used within bone of decreased density and quality.
- OsteoCrete does not result in any untoward effect when placed near, or in contact with, rotator cuff or biceps tendons during fixation procedures.
- OsteoCrete can potentially be used to replace the anchor within tenodesis procedures that utilize transcortical button fixation in addition to anchor fixation.