User login
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Visionary Surgery Saved Pitcher’s Arm. Now Even Children Get It
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Where does it hurt?’: Primary care tips for common ortho problems
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
POISE-3 backs wider use of tranexamic acid in noncardiac surgery
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Shoulder arthroplasty template data require careful interpretation
Proprietary templating software to guide the positioning of total shoulder arthroplasty (TSA) generate very different measures for inclination and version, according to a study that compared four programs and reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled due to COVID-19.
“It is not a question of one software being better than another. They are just different, and they are device specific,” reported Brent B. Wiesel, MD, chief of the shoulder service at the MedStar Georgetown Orthopaedic Institute, Washington.
The variations were substantial and clinically relevant, suggesting that surgeons need to be aware of these differences when switching between the devices, according to Dr. Wiesel. He said that there is no gold standard for positioning total shoulder arthroplasty, which prevents any conclusion about the superiority of one over the other.
In this study, 76 CT scans obtained from shoulders of patients with glenohumeral arthritis were analyzed for native glenoid version and inclination by the ArthrexVIP, Tornier BluePrint, Stryker TrueSight, and ExactechGPS software programs. Dr. Wiesel explained that these are among the most commonly used programs, but there are others.
After extracting the recommended version and inclination measures from each software program, agreement between measures was calculated with an analysis of variance (ANOVA) test. The variance across programs was highly significant for both native glenoid version and inclination (P < .001).
Inter-rater reliability of the software outputs analyzed with Krippendorff’s alpha, for which a value of 1.0 signals perfect agreement and a value of 0 signals complete disagreement, reinforced the discord. For the 76 scans, the values for version and inclination were 0.272 and 0.303, respectively. Both are extremely low.
“The suggested threshold for high reliability is a value of 0.8 or greater,” said Dr. Wiesel, who was contacted about these data after the AAOS annual meeting was canceled. “The lowest acceptable limit for reliability is 0.667 or greater.”
There was disagreement across all programs. The only agreement to reach an acceptable Krippendorff’s alpha was generated by the Tornier BluePrint and Stryker TrueSight programs. These programs modestly agreed on version (0.706 on the Krippendorff’s alpha), but agreement on inclination was below the acceptable threshold.
“In other words, if you take the same scan from the same patient, you will get different angles from these different templating software programs,” Dr. Wiesel said.
There are several messages from these data, according to Dr. Wiesel. In addition to demonstrating the programs generate outputs that do not agree, he suggested that the values provided by the programs should not be considered absolute. Rather, the software values should be interpreted in the context of the individual patient.
“It is easy to get lazy, but it is important to remember that the software is a tool rather than something that will do the procedure for you,” Dr. Wiesel said. He reported that when the software guidance is not consistent with his own experience, he proceeds cautiously.
“On several occasions when the software has provided measures that are not consistent with my own perception, I have not been happy when I went with the software,” he said. “So typically I go with my gut when there is a discrepancy, and the data from this study supports that.”
Because of the difficulty in creating a gold standard for templating when there are multiple variables that influence optimal positioning of components, Dr. Wiesel suggested that “crowd thinking” might eventually determine the values that produce the best results. By crowd thinking, he was referring to Big Data analysis, collating data from a large number of cases performed by a large number of surgeons.
“All of these software programs provide reasonable guidance, but each has different advantages and disadvantages, and it is important to be aware that they are different,” Dr. Wiesel reported.
There are differences in the templating software, and they should be taken into consideration, according to another expert who has looked at this issue. Senior author of a randomized trial evaluating planning strategies for total shoulder arthroplasty ( J Bone Joint Surg AM. 2019:101;446-57), Eric T. Ricchetti, MD, an orthopedic surgeon and director of the shoulder center at the Cleveland Clinic, offered a similar perspective on templating.
“I agree that surgeons should be familiar with the differences that exist in templating software,” Dr. Ricchetti said. Basing his remarks on his own experience and reiterating the conclusion of the AAOS study, he added, “the methods that are used to identify the bone anatomy of the shoulder can vary across software programs, potentially resulting in differences in subsequent measures of glenoid pathology, such as version and inclination, that may impact surgical decision making.”
Dr. Wiesel reports no potential conflicts of interest.
SOURCE: Wiesel B et al. AAOS 2020. Abstract 212.
Proprietary templating software to guide the positioning of total shoulder arthroplasty (TSA) generate very different measures for inclination and version, according to a study that compared four programs and reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled due to COVID-19.
“It is not a question of one software being better than another. They are just different, and they are device specific,” reported Brent B. Wiesel, MD, chief of the shoulder service at the MedStar Georgetown Orthopaedic Institute, Washington.
The variations were substantial and clinically relevant, suggesting that surgeons need to be aware of these differences when switching between the devices, according to Dr. Wiesel. He said that there is no gold standard for positioning total shoulder arthroplasty, which prevents any conclusion about the superiority of one over the other.
In this study, 76 CT scans obtained from shoulders of patients with glenohumeral arthritis were analyzed for native glenoid version and inclination by the ArthrexVIP, Tornier BluePrint, Stryker TrueSight, and ExactechGPS software programs. Dr. Wiesel explained that these are among the most commonly used programs, but there are others.
After extracting the recommended version and inclination measures from each software program, agreement between measures was calculated with an analysis of variance (ANOVA) test. The variance across programs was highly significant for both native glenoid version and inclination (P < .001).
Inter-rater reliability of the software outputs analyzed with Krippendorff’s alpha, for which a value of 1.0 signals perfect agreement and a value of 0 signals complete disagreement, reinforced the discord. For the 76 scans, the values for version and inclination were 0.272 and 0.303, respectively. Both are extremely low.
“The suggested threshold for high reliability is a value of 0.8 or greater,” said Dr. Wiesel, who was contacted about these data after the AAOS annual meeting was canceled. “The lowest acceptable limit for reliability is 0.667 or greater.”
There was disagreement across all programs. The only agreement to reach an acceptable Krippendorff’s alpha was generated by the Tornier BluePrint and Stryker TrueSight programs. These programs modestly agreed on version (0.706 on the Krippendorff’s alpha), but agreement on inclination was below the acceptable threshold.
“In other words, if you take the same scan from the same patient, you will get different angles from these different templating software programs,” Dr. Wiesel said.
There are several messages from these data, according to Dr. Wiesel. In addition to demonstrating the programs generate outputs that do not agree, he suggested that the values provided by the programs should not be considered absolute. Rather, the software values should be interpreted in the context of the individual patient.
“It is easy to get lazy, but it is important to remember that the software is a tool rather than something that will do the procedure for you,” Dr. Wiesel said. He reported that when the software guidance is not consistent with his own experience, he proceeds cautiously.
“On several occasions when the software has provided measures that are not consistent with my own perception, I have not been happy when I went with the software,” he said. “So typically I go with my gut when there is a discrepancy, and the data from this study supports that.”
Because of the difficulty in creating a gold standard for templating when there are multiple variables that influence optimal positioning of components, Dr. Wiesel suggested that “crowd thinking” might eventually determine the values that produce the best results. By crowd thinking, he was referring to Big Data analysis, collating data from a large number of cases performed by a large number of surgeons.
“All of these software programs provide reasonable guidance, but each has different advantages and disadvantages, and it is important to be aware that they are different,” Dr. Wiesel reported.
There are differences in the templating software, and they should be taken into consideration, according to another expert who has looked at this issue. Senior author of a randomized trial evaluating planning strategies for total shoulder arthroplasty ( J Bone Joint Surg AM. 2019:101;446-57), Eric T. Ricchetti, MD, an orthopedic surgeon and director of the shoulder center at the Cleveland Clinic, offered a similar perspective on templating.
“I agree that surgeons should be familiar with the differences that exist in templating software,” Dr. Ricchetti said. Basing his remarks on his own experience and reiterating the conclusion of the AAOS study, he added, “the methods that are used to identify the bone anatomy of the shoulder can vary across software programs, potentially resulting in differences in subsequent measures of glenoid pathology, such as version and inclination, that may impact surgical decision making.”
Dr. Wiesel reports no potential conflicts of interest.
SOURCE: Wiesel B et al. AAOS 2020. Abstract 212.
Proprietary templating software to guide the positioning of total shoulder arthroplasty (TSA) generate very different measures for inclination and version, according to a study that compared four programs and reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled due to COVID-19.
“It is not a question of one software being better than another. They are just different, and they are device specific,” reported Brent B. Wiesel, MD, chief of the shoulder service at the MedStar Georgetown Orthopaedic Institute, Washington.
The variations were substantial and clinically relevant, suggesting that surgeons need to be aware of these differences when switching between the devices, according to Dr. Wiesel. He said that there is no gold standard for positioning total shoulder arthroplasty, which prevents any conclusion about the superiority of one over the other.
In this study, 76 CT scans obtained from shoulders of patients with glenohumeral arthritis were analyzed for native glenoid version and inclination by the ArthrexVIP, Tornier BluePrint, Stryker TrueSight, and ExactechGPS software programs. Dr. Wiesel explained that these are among the most commonly used programs, but there are others.
After extracting the recommended version and inclination measures from each software program, agreement between measures was calculated with an analysis of variance (ANOVA) test. The variance across programs was highly significant for both native glenoid version and inclination (P < .001).
Inter-rater reliability of the software outputs analyzed with Krippendorff’s alpha, for which a value of 1.0 signals perfect agreement and a value of 0 signals complete disagreement, reinforced the discord. For the 76 scans, the values for version and inclination were 0.272 and 0.303, respectively. Both are extremely low.
“The suggested threshold for high reliability is a value of 0.8 or greater,” said Dr. Wiesel, who was contacted about these data after the AAOS annual meeting was canceled. “The lowest acceptable limit for reliability is 0.667 or greater.”
There was disagreement across all programs. The only agreement to reach an acceptable Krippendorff’s alpha was generated by the Tornier BluePrint and Stryker TrueSight programs. These programs modestly agreed on version (0.706 on the Krippendorff’s alpha), but agreement on inclination was below the acceptable threshold.
“In other words, if you take the same scan from the same patient, you will get different angles from these different templating software programs,” Dr. Wiesel said.
There are several messages from these data, according to Dr. Wiesel. In addition to demonstrating the programs generate outputs that do not agree, he suggested that the values provided by the programs should not be considered absolute. Rather, the software values should be interpreted in the context of the individual patient.
“It is easy to get lazy, but it is important to remember that the software is a tool rather than something that will do the procedure for you,” Dr. Wiesel said. He reported that when the software guidance is not consistent with his own experience, he proceeds cautiously.
“On several occasions when the software has provided measures that are not consistent with my own perception, I have not been happy when I went with the software,” he said. “So typically I go with my gut when there is a discrepancy, and the data from this study supports that.”
Because of the difficulty in creating a gold standard for templating when there are multiple variables that influence optimal positioning of components, Dr. Wiesel suggested that “crowd thinking” might eventually determine the values that produce the best results. By crowd thinking, he was referring to Big Data analysis, collating data from a large number of cases performed by a large number of surgeons.
“All of these software programs provide reasonable guidance, but each has different advantages and disadvantages, and it is important to be aware that they are different,” Dr. Wiesel reported.
There are differences in the templating software, and they should be taken into consideration, according to another expert who has looked at this issue. Senior author of a randomized trial evaluating planning strategies for total shoulder arthroplasty ( J Bone Joint Surg AM. 2019:101;446-57), Eric T. Ricchetti, MD, an orthopedic surgeon and director of the shoulder center at the Cleveland Clinic, offered a similar perspective on templating.
“I agree that surgeons should be familiar with the differences that exist in templating software,” Dr. Ricchetti said. Basing his remarks on his own experience and reiterating the conclusion of the AAOS study, he added, “the methods that are used to identify the bone anatomy of the shoulder can vary across software programs, potentially resulting in differences in subsequent measures of glenoid pathology, such as version and inclination, that may impact surgical decision making.”
Dr. Wiesel reports no potential conflicts of interest.
SOURCE: Wiesel B et al. AAOS 2020. Abstract 212.
FROM AAOS 2020
Simple prevention strategies can lessen postoperative delirium after orthopedic surgery
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
FROM BMC GERIATRICS
Fragility Fractures: Diagnosis and Treatment
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
TAKE-HOME POINTS
- 3 million people sustain fragility fractures annually, and nearly 30% die within a year of the fracture.
- The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.
- The World Health Organization has developed a set of T-core criteria to diagnose osteoporosis in postmenopausal women: a score >–1 is normal; <–1 but >–2.5 signifies osteopenia; <–2.5 denotes osteoporosis; and <–2.5 with fragility fracture indicates severe osteoporosis.
- The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children. The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender. Z scores <–2.0 indicate low BMD for chronological age. A Z score > –2.0 is considered within the expected range for age.
- After an initial fragility fracture, the risk for additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture.26
The Characteristics of Surgeons Performing Total Shoulder Arthroplasty: Volume Consistency, Training, and Specialization
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.
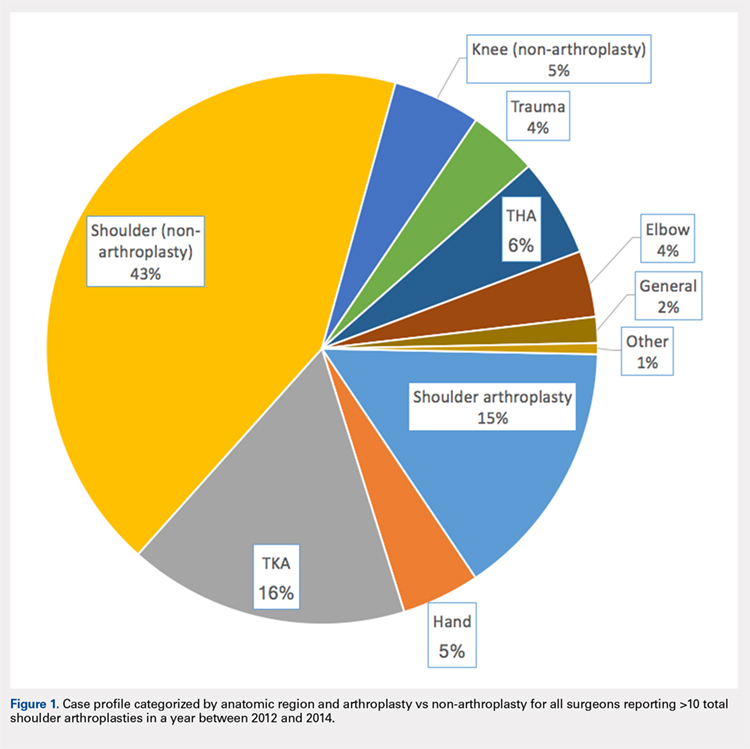
Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.
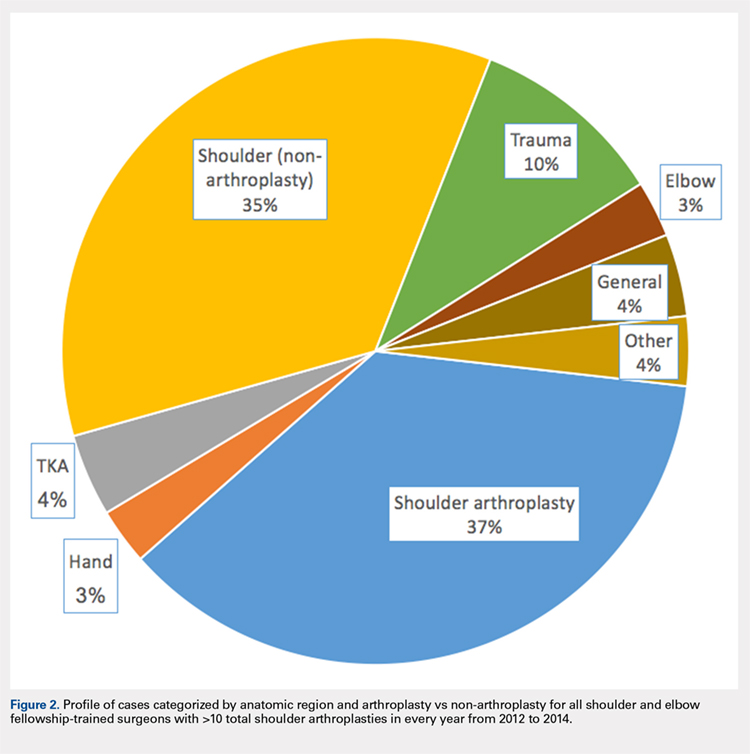
Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.

Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.

Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.

Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.

Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
TAKE-HOME POINTS
- Between 2012 and 2014, 1,374 surgeons performed >10 total shoulder arthroplasties (TSA) in Medicare patients in at least one year.
- From 2012 to 2014, the number of surgeons performing at least 10 TSA in Medicare patients increased from 834 to 1,078, while the number of TSA increased from 21,137 (25.3 per surgeon) to 26,765 (24.9 per surgeon).
- Many of these surgeons had a diverse surgical practice, with nearly one-half performing total knee arthroplasty, one-third performing non-arthroplasty knee surgeries, and >80% performing non-arthroplasty shoulder procedures.
- Only one-third of these surgeons had formal fellowship training specific to shoulder and elbow.
- In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each currently practicing fellowship graduate would have to perform 140.6 TSA in Medicare patients annually.