User login
Massive Rotator Cuff Tears in Patients Older Than Sixty-five: Indications for Cuff Repair versus Reverse Total Shoulder Arthroplasty
ABSTRACT
The decision to perform rotator cuff repair (RCR) versus reverse total shoulder arthroplasty (rTSA) for massive rotator cuff tear (MCT) without arthritis can be difficult. Our aim was to identify preoperative variables that are influential in a surgeon's decision to choose one of the two procedures and evaluate outcomes.
We retrospectively reviewed 181 patients older than 65 who underwent RCR or rTSA for MCT without arthritis. Clinical and radiographic data were collected and used to evaluate the preoperative variables in each of these two patient populations and assess outcomes.
Ninety-five shoulders underwent RCR and 92 underwent rTSA with an average followup of 44 and 47 months, respectively. Patients selected for RCR had greater preoperative flexion (113 vs 57), abduction (97 vs 53), and external rotation (42 vs 32), higher SST (3.1 vs 1.9) and ASES scores (43.8 vs 38.6), and were less likely to have had previous cuff surgery (6.3% vs 35.9%). Patients selected for rTSA had a smaller acromiohumeral interval (4.8 vs 8.7) and more superior subluxation (50.6% vs 14.1%). Similar preoperative characteristics included pain, comorbidities, and BMI. Patients were satisfied in both groups and had significant improvement in motion and function postoperatively.
Both RCR and rTSA can result in significant functional improvement and patient satisfaction in the setting of MCT without arthritis in patients older than 65. At our institution, patients who underwent rTSA had less pre-operative motion, lower function, more evidence of superior migration, and were more likely to have had previous rotator cuff surgery.
Continue to: The treatment of patients...
The treatment of patients with massive rotator cuff tears (MCTs) without osteoarthritis is challenging. This population is of considerable interest, as the prevalence of MCT has been reported to be as high as 40% of all rotator cuff tears.1Options for surgical treatment in patients who have failed conservative management are numerous and include tendon debridement, partial or complete arthroscopic or open rotator cuff repair (RCR), tendon transfers, reverse total shoulder arthroplasty (rTSA), arthroscopic superior capsular reconstruction (ASCR), and other grafting procedures.2 Arthroscopic superior capsular reconstruction shows promise as a novel technique, but it is not yet well studied. Other procedures such as tendon transfers fit into the treatment algorithm for only a small subset of patients. Open rotator cuff repair and rTSA are the 2 most commonly utilized procedures for MCT, and both have been shown to reliably achieve significant functional improvement and patient satisfaction.3–6
The dilemma for the treating surgeon is deciding which patients to treat with RCR and who to treat with rTSA. Predicting which surgical procedure will provide a better functional result is difficult and controversial.7 The RCR method is a bone-conserving procedure with relatively low surgical risk and allows the option for rTSA to be performed as a salvage surgery should repair fail. It also may be less costly in the appropriate population.8 However, large rotator cuff tears in elderly patients have low healing potential, and the prospect of participating in a lengthy rehabilitation after an operation that may not prove successful can be deterring.9,10 In the elderly population, rTSA may be a reliable option, as tendon healing of the cuff is not necessary to restore function. However, rTSA does not conserve bone, provides a non-anatomic solution, and has had historically high complication rates.4,5
In an effort to aid in the decision-making process when considering these 2 surgical options, we compared RCR and rTSA performed at a single institution for MCT in patients >65 years. Our aim was to identify preoperative patient variables that influence a surgeon’s decision to proceed with 1 of the 2 procedures. Moreover, we evaluated clinical outcomes in these 2 patient populations. We hypothesized that (1) patients selected for rTSA would have worse preoperative function, less range of motion, more comorbidities, more evidence of radiographic subluxation, and a higher likelihood of having undergone previous RCR than those selected for RCR, and (2) both RCR and rTSA would be successful and result in improved clinical outcomes with high patient satisfaction.
MATERIALS AND METHODS
PATIENT SELECTION
We performed a retrospective chart review using our practice database of all patients undergoing arthroscopic RCR and rTSA for any indication by the senior author (M.A.F.) between January 2004 and April 2015. A total of 1503 RCRs and 1973 rTSAs were conducted during the study period. Patient medical records were reviewed, and those meeting the following criteria were included in the study: >65 years at the time of surgery, MCT, no preoperative glenohumeral arthritis, minimum follow-up of 12 months, functional deltoid muscle on physical examination, and no prior shoulder surgery except for RCR or diagnostic arthroscopy. A total of 92 patients who underwent arthroscopic RCR and 89 patients who underwent rTSA met the inclusion criteria. For patients with bilateral shoulder surgery, we measured each shoulder independently. Three patients underwent bilateral rTSA, and 3 patients underwent bilateral RCR, leaving 95 shoulders in the RCR group and 92 in the rTSA group. The Western Institutional Review Board determined this study to be exempt from review.
RADIOGRAPHIC EVALUATION
All patient charts included a radiology report and documented interpretation of the images by the treating surgeon prior to surgery. Radiographs were assessed to assure the absence of preoperative glenohumeral osteoarthritis. The images were also graded based on the Hamada classification.11 Stage 1 is associated with minimal radiographic change with an acromiohumeral interval (AHI) >6 mm; stage 2 is characterized by narrowing of the AHI <6 mm; and Stage 3 is defined by narrowing of the AHI with radiographic changes of the acromion. Stages 4 and higher include arthritic changes to the glenohumeral joint, and they were not included in the study population. The AHI measurements and the presence or absence of glenohumeral subluxation were documented.
Continue to: MASSIVE CUFF TEAR DETERMINATION...
MASSIVE CUFF TEAR DETERMINATION
We defined MCT on the basis of previously described criteria of tears involving ≥2 tendons or tears measuring ≥5 cm in greatest dimension.12,13 Patient charts were screened, and those whose clinical notes or radiology reports indicated an absence of MCT were excluded. Preoperative imaging of the remaining patients was then evaluated by 3 fellowship-trained shoulder surgeons to confirm MCT in all patients with a clinically documented MCT, as well as to assess those who had insufficient documentation of tear size in the notes.
Advanced imaging was evaluated for fatty atrophy of the rotator cuff musculature, and Goutallier classification was assigned.14,15 Length of retraction was measured from the tendon end to the medial aspect of the footprint on coronal imaging, and the subscapularis and teres minor were assessed and documented as torn or intact.16,17
DATA COLLECTION
We reviewed clinical charts and patient questionnaire forms from both the preoperative and follow-up visits. Clinical data collected included gender, age at surgery, active range of motion (forward elevation, abduction, external rotation, and internal rotation), comorbidities, smoking status, BMI, history of shoulder surgery, and any postoperative complications or need for secondary surgery. All patients completed patient-centered questionnaires regarding shoulder pain and dysfunction at each visit or via telephone communication with clinic staff. Outcome measurements used for analysis included American Shoulder and Elbow Surgeons (ASES) Score, simple shoulder test (SST), visual analog score (VAS) pain scale, and patient-reported satisfaction (Graded 1-10; 1 = poor outcome; 4 = satisfactory outcome; 7 = good outcome; 10 = excellent outcome).
DATA ANALYSIS AND STATISTICAL METHODS
Statistical tests were selected based on the result of Shapiro–Wilk test for normality. Continuous variables were evaluated with either independent t test or Mann–Whitney U test. Dependent t test was used to evaluate outcome variables. For categorical variables, either Pearson’s χ2or Fisher’s exact test was performed depending on the sample size. Alpha was set at P =.05.
Continue to: RESULTS...
RESULTS
PREOPERATIVE CHARACTERISTICS
Of the 187 shoulders in the study group, 95 had RCR and 92 had rTSA. Demographic information and preoperative variables for both groups are summarized in Table 1 and Table 2. Patients in the RCR group had greater preoperative forward elevation, abduction, and external rotation and higher preoperative functional scores than those in the rTSA group. Patients in the rTSA group were older and more likely to be female than those in the RCR group. More patients in the rTSA group had undergone prior RCR compared with those in the RCR group. Each of these differences was statistically significant. Subjective pain scores, BMI, and comorbidities were similar between the 2 groups.
Table 1. Patient demographics
| RCR | rTSA | P value |
Age (yr; mean ± SD) | 71 ± 5 | 74 ± 6 | <.0001 |
Gender *male (no.; %) *female (no.; %) | 57 (60%) 38 (40%) | 30 (33%) 62 (67%) | <.0001 |
BMI (mean ± SD) | 28.5 ± 4.4 | 28.1 ± 4.5 | .578 |
Abbreviations: BMI, body mass index; RCR, rotator cuff repair; rTSA, reverse total shoulder arthroplasty.
Table 2. Preoperative variables
| RCR (n=95) | rTSA (n=92) | P value |
Radiographic parameters | |||
AB interval | 9 ± 3 | 5 ± 3 | <.0001 |
Humeral escape | 14.1% | 50.6% | <.0001 |
Hamada 1 | 76.1% | 15.6% | <.0001 |
Hamada 2 | 13.0% | 50.6% | |
Hamada 3 | 10.9% | 33.8% | |
Goutallier grade 1 | 7.8% | 19.3% | .227 |
Goutallier grade 2 | 66.7% | 52.6% | |
Goutallier grade 3 | 21.6% | 19.3% | |
Goutallier grade 4 | 3.9% | 8.8% | |
Clinical measures | |||
Preop FE | 113 ± 50 | 57 ± 34 | <.0001 |
Preop AB | 97 ± 45 | 53 ± 35 | <.0001 |
Preop ER | 42 ± 25 | 32 ± 28 | .029 |
Preop IR | 2.9 ± 1.6 | 2.6 ± 1.8 | .247 |
Preop pain | 5.7 ± 2.3 | 5.6 ± 2.5 | .927 |
Preop ASES | 44 ± 17 | 39 ± 16 | .04 |
Preop SST | 3.1 ± 2.6 | 1.9 ± 1.7 | .001 |
Patients parameters | |||
Previous cuff surgery | 6.3% | 35.9% | <.0001 |
Comorbidity count | 1.7 ± 1.4 | 2.1 ± 2.7 | .126 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; preop, preoperative; SST, simple shoulder test.
Radiographically, patients selected to undergo rTSA had a smaller AHI (4.8 vs 8.7, P < .0001) and more evidence of superior subluxation (50.6% vs 14.1%, P < .0001) than those in the RCR group. Average Hamada grade was 1.4 ± 0.7 and 2.2 ± 0.7 for the RCR and rTSA groups, respectively (P < .0001). Average Goutallier grade was similar between the groups (2.2 ± 0.6 for RCR vs 2.2 ± 0.8 for rTSA, P =.227), and 25.5% of the RCR group had Grade 3 or 4 atrophy compared with 28.1% of the rTSA group.
POSTOPERATIVE OUTCOMES
The average follow-up time was 44 months for RCR and 47 months for rTSA. Patients in the RCR and rTSA groups were highly satisfied with the surgery (8.5 ± 2.6 vs 8.2 ± 2.6, P = .461) and had significantly increased range of motion in all planes and improved functional scores (Table 3). The rTSA group had greater net improvement in forward elevation, abduction, and external rotation than the RCR group. Both groups demonstrated similar improvement in ASES, SST, and VAS pain scores.
Table 3. Postoperative outcomes
| RCR (n=95) | P value | rTSA (n=92) | P value | ||
Preoperative | Postoperative | Preoperative | Postoperative | |||
FE | 113 ± 50 | 166 ± 26 | <.0001 | 57 ± 34 | 136 ± 46 | <.0001 |
AB | 97 ± 45 | 155 ± 37 | <.0001 | 53 ± 35 | 129 ± 44 | <.0001 |
ER | 42 ± 25 | 48 ± 20 | .033 | 32 ± 28 | 57 ± 32 | <.0001 |
IR | 2.9 ± 1.6 | 4.6 ± 1.6 | <.0001 | 2.6 ± 1.8 | 4.7 ± 2.4 | <.0001 |
VAS pain | 5.7 ± 2.3 | 1.7 ± 2.4 | <.0001 | 5.6 ± 2.5 | 1.6 ± 2.5 | <.0001 |
ASES | 44 ± 17 | 83 ± 18 | <.0001 | 39 ± 16 | 77 ± 22 | <.0001 |
SST | 3.1 ± 2.6 | 9.3 ± 2.9 | <.0001 | 1.9 ± 1.7 | 7.1 ± 3.4 | <.0001 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; SST, simple shoulder test; VAS – visual analog score.
In the RCR group, 5 patients (5.3%) required reoperation: 3 patients underwent conversion to rTSA, 1 patient underwent biceps tenotomy with subacromial decompression, and 1 patient underwent arthroscopic irrigation and debridement for a postoperative Propionibacterium acnes infection. In the rTSA group, 2 patients (2.2%) required reoperation: 1 patient underwent open reduction internal fixation for a scapula fracture that failed conservative management, and 1 patient had an open irrigation and debridement with polyethylene exchange for an acute postoperative infection of unknown source.
DISCUSSION
Massive, retracted rotator cuff tears are a common and difficult problem.1 The treatment options are numerous and depend on a variety of preoperative factors including patient-specific characteristics and factors specific to the tear. For certain patients, nonoperative management may be a reasonable first step, as an MCT does not necessarily preclude painless, functional shoulder motion. Elderly, lower demand individuals have been shown to do well with physical rehabilitation.18 Similarly, for the same category of elderly patients who do not respond to conservative measures, arthroscopic tendon debridement with or without subacromial decompression and/or biceps tenotomy may be effective.1,19 This technique has been described as “limited goals surgery;” despite some mixed results in the literature, multiple studies have reported symptomatic and functional improvement after simple debridement.2,19–21The consensus among several authors has been that this procedure continues to play a role for elderly, low-demand patients whose functional goals are limited and whose primary complaint is pain.1,2,20
For the majority of patients with MCT who desire pain relief and a restoration of shoulder function, RCR remains the gold standard of treatment and should be the primary aim if feasible. Complete RCR has consistently outperformed both partial repair and debridement in multiple studies in terms of pain relief and functional improvement.10,21,22However, elderly patients with chronic, massive tears, particularly in the setting of muscle atrophy, are at high risk of failure with attempted cuff repair.9,23 Novel techniques such as superior capsular reconstruction and subacromial balloon spacer implantation may offer a minimally invasive method of re-centering the humeral head and stabilizing the glenohumeral joint; however, these new treatment options lack any long-term data in the literature to support their widespread use.24–26 Alternatively, rTSA has been shown to be a reliable option to restore shoulder function in the setting of a massive irreparable rotator cuff tear, even in the absence of arthritis.5,27-31
Continue to: The decision-making process...
The decision-making process for selecting RCR or rTSA in the setting of MCT without arthritis in the older population (age >65 years) remains challenging. We attempted to quantify the data of a high-volume surgeon and identify the differences and similarities between those patients selected for either procedure. At our institution, we generally performed rTSA on patients with low preoperative range of motion, poor function based on SST and ASES scores, small AHI, and strong evidence of superior subluxation. We were also more likely to perform rTSA if the patient had a history of rotator cuff surgery. There was a predilection for older age and female gender in those who underwent rTSA.
For our study, we elected to focus on patients >65 years. In our experience, the choice of which surgical procedure to perform is generally easier in younger patients. Most surgeons appropriately opt for an arthroscopic procedure or tendon transfer to preserve bone and maintain the option of rTSA as a salvage procedure if necessary in the future. Studies have reported that <60 years is a predictor of poor outcome with rTSA, and patients <65 years who undergo rTSA have been shown to have high complication rates.30-32 Furthermore, the longevity of the implant in young patients is a significant concern, and revision surgery after rTSA is technically demanding and known to result in poor functional outcomes.32,33
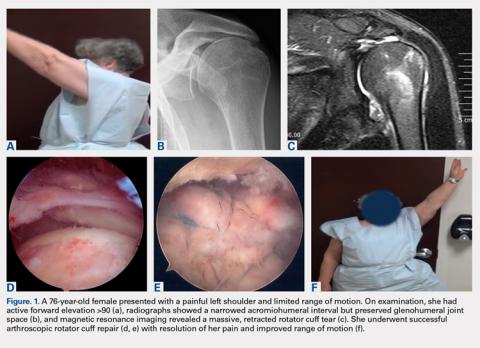
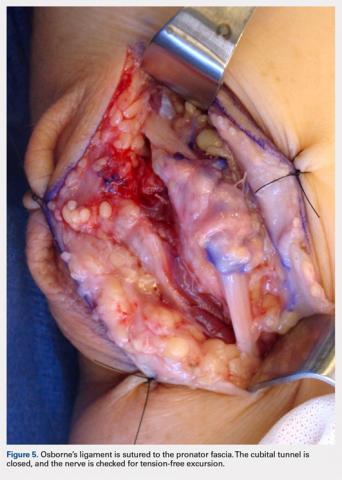
Although the indications for rTSA are expanding, attempts at RCR in the setting of MCT remain largely appropriate. Preserved preoperative anterior elevation >90° has been associated with loss of motion after rTSA and poor satisfaction, and one should exercise caution when considering rTSA in this setting.3 The current study confirmed that even older patients with MCT may be very satisfied with arthroscopic RCR (Figure 1). Both range of motion and function significantly improved, and patients were largely satisfied with the procedure with an average self-reported outcome of good to excellent. At the time of final follow-up for this study, only 3 shoulders in the RCR group had undergone conversion to rTSA. This number may be expected to rise with long follow-up periods, and we feel that prolonging the time before arthroplasty is generally in the best interest of the patient.
Our results were consistent with several reported studies in which RCR has been shown to be successful in the setting of MCT.34–37 Henry and colleagues36 performed a systematic review that evaluated 954 patients who underwent partial or complete anatomic RCR for MCT. Although the average age was 63 years (range, 37–87), functional outcome scores, VAS pain score, and overall range of motion consistently and significantly improved.
rTSA may be a “more reliable” option than RCR in treating MCT in the older population because it does not rely on tendon healing. However, the relationship between tendon healing and clinical outcomes after RCR is unclear. The aforementioned systematic review reported re-tear rates to be as high as 79%, but several studies have reported high satisfaction even in the setting of retear.36 Yoo and colleagues38 and Chung and colleagues9 reported re-tear rates of 45.5% and 39.8%, respectively, but both studies noted that there was no difference in outcome measures between those patients with and without re-tears. In particular, for patients who have had no prior rotator cuff surgery, an attempt at arthroscopic repair may be a prudent option with relatively low risk.
Although certain patients may clinically improve despite suffering a re-tear (or inability to heal in the first place), others continue to experience pain and dysfunction that negatively affect their quality of life.39–41 These patients are more often appropriate candidates for rTSA. Indeed, several studies have demonstrated a higher re-tear rate in patients with a history of surgery than in those without.23,31,38,42 Shamsudin and colleagues43 found revision arthroscopic RCR, even in a younger age group with tears of all sizes, to be twice as likely to re-tear. Notably, re-tear after revision repair may be more likely to be symptomatic, as these re-tears are routinely associated with pain, stiffness, and loss of function. Even in the hands of experienced surgeons in a younger population, revision repair has only been able to reverse pseudoparalysis in 43% of patients, leading to only 39% return to sport or full activity.44 In examining our data, we were much less likely to perform an RCR in patients who had a history of cuff repair surgery than in those without this history.
Continue to: Overall, those patients selected for rTSA...
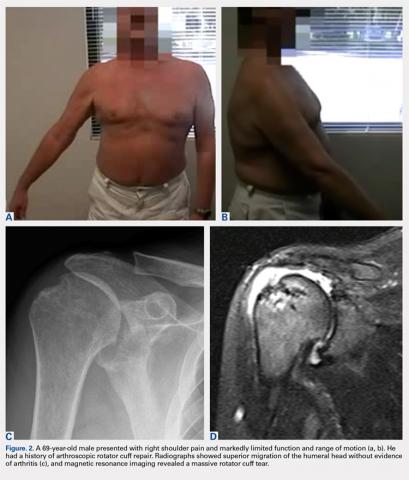
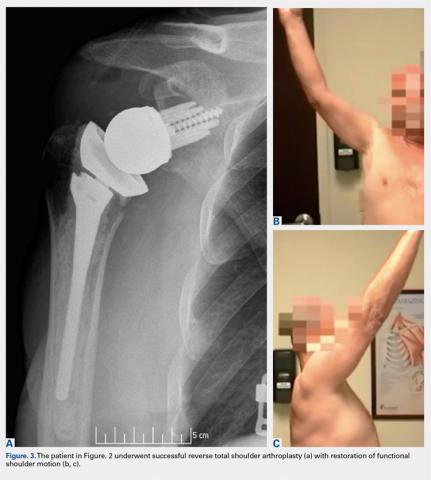
Overall, those patients selected for rTSA in our study population performed well postoperatively (Figure 2 and Figure 3). Vast improvements were noted in range of motion, function, and pain scores at final follow up. Moreover, no patients in the study group required revision arthroplasty during the follow-up period. Although the average follow-up period was only 47 months, these results suggested that elderly patients with MCT without arthritis may be particularly ideal candidates for rTSA with regard to implant survival and anticipated revision rate when chosen appropriately.
Several weaknesses were noted within this paper. First, the study was retrospective, precluding randomization of treatment groups and standardization of data collection and follow-up. The outcomes of RCR and rTSA could not be compared directly due to the inherent selection bias. The groups clearly differed in many respects, and these preoperative factors likely played a role in postoperative outcomes. However, the primary goal of this study was not to compare outcomes of the treatment groups but to analyze the patterns of patient selection by an experienced treating surgeon and contribute to published data that each surgery can be successful in this patient population when chosen appropriately.
Second, our data were based on a single surgeon’s decisions, and results may not be generalizable. Furthermore, the senior author has had a longstanding interest in reverse shoulder arthroplasty and has published data illustrating successful outcomes for rTSA in patients with MCT. For this reason, one could presume that there may have been some bias toward treating patients with rTSA. However, we feel that the senior author’s unique and longstanding experience in treating MCT allows for a thorough evaluation and comparison of preoperative variables and outcomes declared within this study. Indeed, many patients included in this study were referred from outside institutions specifically for rTSA but instead were deemed more appropriate candidates for RCR and underwent successful arthroscopic repair, a common scenario which served as an impetus for this study.
CONCLUSION
RCR and rTSA are both viable options for patients >65 years with MCT without arthritis. Treatment must be individualized for each patient with careful consideration of a number of preoperative variables and patient characteristics. At our institution, patients with previous RCR, decreased range of motion, poor function, and strong radiographic evidence of subluxation are more likely to undergo rTSA. When chosen appropriately, both RCR and rTSA can result in improved range of motion, function, and high patient satisfaction in this patient population.
- Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894-1908. doi:10.2106/JBJS.I.01531.
- Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493-1505. doi:10.1016/j.jse.2015.04.005.
- Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600-606. doi:10.1016/j.jse.2009.03.011.
- Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244-1251. doi:10.2106/JBJS.G.00775.
- Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544-2556.doi:10.2106/JBJS.I.00912.
- Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476-1485. doi:10.2106/JBJS.F.00666.
- Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
- Makhni EC, Swart E, Steinhaus ME, Mather RC 3rd, Levine WN, Bach BR Jr et al. Cost-effectiveness of reverse total shoulder arthroplasty versus arthroscopic rotator cuff repair for symptomatic large and massive rotator cuff tears. Arthroscopy. 2016;32(9):1771-1780. doi:10.1016/j.arthro.2016.01.063.
- Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41:1674-1683. doi:10.1177/0363546513485719.
- Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180. doi:10.1186/1471-2474-15-180.
- Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
- DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563-567.
- Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505-515.
- Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599-605.
- Goutallier D, Bernageau J, Patte D. Assessment of the trophicity of the muscles of the ruptured rotator cuff by CT scan. In: Post M, Morrey B, Hawkins R, eds. Surgery of the Shoulder. St. Louis, MO: Mosby, 1990;11-13.
- Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
- Meyer DC, Wieser K, Farshad M, Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40:2242-2247.
- Williams GR Jr, Rockwood CA Jr, Bigliani LU, Ianotti JP, Stanwood W. Rotator cuff tears: why do we repair them? J Bone Joint Surg Am. 2004;86-A(12):2764-2776.
- Rockwood CA Jr, Williams GR Jr, Burkhead WZ Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857-866.
- Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthopaed Traumatol. 2010;11:13-20. doi 10.1007/s10195-010-0084-0.
- Heuberer PR, Kolblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24:3828-3837.
- Moser M, Jablonski MV, Horodyski M, Wright TW. Functional outcome of surgically treated massive rotator cuff tears: a comparison of complete repair, partial repair, and debridement. Orthopedics.2007;30(6):479-482.
- Rhee YG, Cho NS, Yoo JH. Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy. 2014;30:546-554. doi:10.1016/j.arthro.2014.02.006.
- Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93-99. doi: 10.1016/j.arthro.2017.08.265.
- Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy.2013;29:459-70.
- Piekaar RSM, Bouman ICE, van Kampen PM, van Eijk F, Huijsmans PE. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskelet Surg. 2018;102(3):247-255. doi: 10.1007/s12306-017-0525-5.
- Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23:1662-1668. doi:10.1016/j.jse.2014.03.001.
- Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S-161S. doi:10.1016/j.jse.2004.10.006.
- Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-68. doi: 10.3928/0147-7447-19930101-11.
- Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24:1698-1706. doi:10.1016/j.jse.2015.04.015.
- Kim HM, Caldwell JM, Buza JA, Fink LA, Ahmad CS, Bigliani LU, Levine WN. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96:106-112. doi:10.2106/JBJS.L.01649.
- Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208. doi:10.1016/j.jse.2012.11.016.
- Sershon RA, Van Thiel GS, Lin EC, McGill KC, Cole BJ, Verma NN, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg.2014;23:395-400. doi:10.1016/j.jse.2013.07.047.
- Denard PJ, Ladermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28:1214-1219. doi:10.1016/j.arthro.2012.02.026.
- Denard PJ, Ladermann A, Brady PC, Narbona P, Adams CR, Arrigoni P, et al. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med. 2015;43:2373-2378. doi: 10.1177/0363546515597486.
- Henry P, Wasserstein D, Park S, Dwyer T, Chahal J, Slobogean G, Schemitsch E. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31:2472-2480. doi:10.1016/j.arthro.2015.06.038.
- Oh JH, Kim SH, Shin SH, Chung SW, Kim JY, Kim SJ. Outcome of rotator cuff repair in large-to-massive tear with pseudoparalysis: a comparative study with propensity score matching. Am J Sports Med.2011;39:1413-1420.
- Yoo JC, Ahn JH, Koh KH, Lim KS. Rotator cuff integrity after arthroscopic repair for large tears with less-than-optimal footprint coverage. Arthroscopy. 2009;25:1093-1100. doi:10.1016/j.arthro.2009.07.010.
- Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304-314.
- Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, Flatow EL Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. Am J Sports Med. 2004;32:1716-1722.
- Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10:54-60.
- Papadopoulos P, Karataglis D, Boutsiadis A, Fotiadou A, Christoforidis J, Christodoulou A. Functional outcome and structural integrity following mini-open repair of large and massive rotator cuff tears: a 3-5 year follow-up study. J Shoulder Elbow Surg. 2011;20:131-137. doi:10.1016/j.jse.2010.05.026.
- Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med.2015;43:557-564. doi:10.1177/0363546514560729.
- Ladermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy. 2011;27:1620-1627. doi:10.1016/j.arthro.2011.08.290.
ABSTRACT
The decision to perform rotator cuff repair (RCR) versus reverse total shoulder arthroplasty (rTSA) for massive rotator cuff tear (MCT) without arthritis can be difficult. Our aim was to identify preoperative variables that are influential in a surgeon's decision to choose one of the two procedures and evaluate outcomes.
We retrospectively reviewed 181 patients older than 65 who underwent RCR or rTSA for MCT without arthritis. Clinical and radiographic data were collected and used to evaluate the preoperative variables in each of these two patient populations and assess outcomes.
Ninety-five shoulders underwent RCR and 92 underwent rTSA with an average followup of 44 and 47 months, respectively. Patients selected for RCR had greater preoperative flexion (113 vs 57), abduction (97 vs 53), and external rotation (42 vs 32), higher SST (3.1 vs 1.9) and ASES scores (43.8 vs 38.6), and were less likely to have had previous cuff surgery (6.3% vs 35.9%). Patients selected for rTSA had a smaller acromiohumeral interval (4.8 vs 8.7) and more superior subluxation (50.6% vs 14.1%). Similar preoperative characteristics included pain, comorbidities, and BMI. Patients were satisfied in both groups and had significant improvement in motion and function postoperatively.
Both RCR and rTSA can result in significant functional improvement and patient satisfaction in the setting of MCT without arthritis in patients older than 65. At our institution, patients who underwent rTSA had less pre-operative motion, lower function, more evidence of superior migration, and were more likely to have had previous rotator cuff surgery.
Continue to: The treatment of patients...
The treatment of patients with massive rotator cuff tears (MCTs) without osteoarthritis is challenging. This population is of considerable interest, as the prevalence of MCT has been reported to be as high as 40% of all rotator cuff tears.1Options for surgical treatment in patients who have failed conservative management are numerous and include tendon debridement, partial or complete arthroscopic or open rotator cuff repair (RCR), tendon transfers, reverse total shoulder arthroplasty (rTSA), arthroscopic superior capsular reconstruction (ASCR), and other grafting procedures.2 Arthroscopic superior capsular reconstruction shows promise as a novel technique, but it is not yet well studied. Other procedures such as tendon transfers fit into the treatment algorithm for only a small subset of patients. Open rotator cuff repair and rTSA are the 2 most commonly utilized procedures for MCT, and both have been shown to reliably achieve significant functional improvement and patient satisfaction.3–6
The dilemma for the treating surgeon is deciding which patients to treat with RCR and who to treat with rTSA. Predicting which surgical procedure will provide a better functional result is difficult and controversial.7 The RCR method is a bone-conserving procedure with relatively low surgical risk and allows the option for rTSA to be performed as a salvage surgery should repair fail. It also may be less costly in the appropriate population.8 However, large rotator cuff tears in elderly patients have low healing potential, and the prospect of participating in a lengthy rehabilitation after an operation that may not prove successful can be deterring.9,10 In the elderly population, rTSA may be a reliable option, as tendon healing of the cuff is not necessary to restore function. However, rTSA does not conserve bone, provides a non-anatomic solution, and has had historically high complication rates.4,5
In an effort to aid in the decision-making process when considering these 2 surgical options, we compared RCR and rTSA performed at a single institution for MCT in patients >65 years. Our aim was to identify preoperative patient variables that influence a surgeon’s decision to proceed with 1 of the 2 procedures. Moreover, we evaluated clinical outcomes in these 2 patient populations. We hypothesized that (1) patients selected for rTSA would have worse preoperative function, less range of motion, more comorbidities, more evidence of radiographic subluxation, and a higher likelihood of having undergone previous RCR than those selected for RCR, and (2) both RCR and rTSA would be successful and result in improved clinical outcomes with high patient satisfaction.
MATERIALS AND METHODS
PATIENT SELECTION
We performed a retrospective chart review using our practice database of all patients undergoing arthroscopic RCR and rTSA for any indication by the senior author (M.A.F.) between January 2004 and April 2015. A total of 1503 RCRs and 1973 rTSAs were conducted during the study period. Patient medical records were reviewed, and those meeting the following criteria were included in the study: >65 years at the time of surgery, MCT, no preoperative glenohumeral arthritis, minimum follow-up of 12 months, functional deltoid muscle on physical examination, and no prior shoulder surgery except for RCR or diagnostic arthroscopy. A total of 92 patients who underwent arthroscopic RCR and 89 patients who underwent rTSA met the inclusion criteria. For patients with bilateral shoulder surgery, we measured each shoulder independently. Three patients underwent bilateral rTSA, and 3 patients underwent bilateral RCR, leaving 95 shoulders in the RCR group and 92 in the rTSA group. The Western Institutional Review Board determined this study to be exempt from review.
RADIOGRAPHIC EVALUATION
All patient charts included a radiology report and documented interpretation of the images by the treating surgeon prior to surgery. Radiographs were assessed to assure the absence of preoperative glenohumeral osteoarthritis. The images were also graded based on the Hamada classification.11 Stage 1 is associated with minimal radiographic change with an acromiohumeral interval (AHI) >6 mm; stage 2 is characterized by narrowing of the AHI <6 mm; and Stage 3 is defined by narrowing of the AHI with radiographic changes of the acromion. Stages 4 and higher include arthritic changes to the glenohumeral joint, and they were not included in the study population. The AHI measurements and the presence or absence of glenohumeral subluxation were documented.
Continue to: MASSIVE CUFF TEAR DETERMINATION...
MASSIVE CUFF TEAR DETERMINATION
We defined MCT on the basis of previously described criteria of tears involving ≥2 tendons or tears measuring ≥5 cm in greatest dimension.12,13 Patient charts were screened, and those whose clinical notes or radiology reports indicated an absence of MCT were excluded. Preoperative imaging of the remaining patients was then evaluated by 3 fellowship-trained shoulder surgeons to confirm MCT in all patients with a clinically documented MCT, as well as to assess those who had insufficient documentation of tear size in the notes.
Advanced imaging was evaluated for fatty atrophy of the rotator cuff musculature, and Goutallier classification was assigned.14,15 Length of retraction was measured from the tendon end to the medial aspect of the footprint on coronal imaging, and the subscapularis and teres minor were assessed and documented as torn or intact.16,17
DATA COLLECTION
We reviewed clinical charts and patient questionnaire forms from both the preoperative and follow-up visits. Clinical data collected included gender, age at surgery, active range of motion (forward elevation, abduction, external rotation, and internal rotation), comorbidities, smoking status, BMI, history of shoulder surgery, and any postoperative complications or need for secondary surgery. All patients completed patient-centered questionnaires regarding shoulder pain and dysfunction at each visit or via telephone communication with clinic staff. Outcome measurements used for analysis included American Shoulder and Elbow Surgeons (ASES) Score, simple shoulder test (SST), visual analog score (VAS) pain scale, and patient-reported satisfaction (Graded 1-10; 1 = poor outcome; 4 = satisfactory outcome; 7 = good outcome; 10 = excellent outcome).
DATA ANALYSIS AND STATISTICAL METHODS
Statistical tests were selected based on the result of Shapiro–Wilk test for normality. Continuous variables were evaluated with either independent t test or Mann–Whitney U test. Dependent t test was used to evaluate outcome variables. For categorical variables, either Pearson’s χ2or Fisher’s exact test was performed depending on the sample size. Alpha was set at P =.05.
Continue to: RESULTS...
RESULTS
PREOPERATIVE CHARACTERISTICS
Of the 187 shoulders in the study group, 95 had RCR and 92 had rTSA. Demographic information and preoperative variables for both groups are summarized in Table 1 and Table 2. Patients in the RCR group had greater preoperative forward elevation, abduction, and external rotation and higher preoperative functional scores than those in the rTSA group. Patients in the rTSA group were older and more likely to be female than those in the RCR group. More patients in the rTSA group had undergone prior RCR compared with those in the RCR group. Each of these differences was statistically significant. Subjective pain scores, BMI, and comorbidities were similar between the 2 groups.
Table 1. Patient demographics
| RCR | rTSA | P value |
Age (yr; mean ± SD) | 71 ± 5 | 74 ± 6 | <.0001 |
Gender *male (no.; %) *female (no.; %) | 57 (60%) 38 (40%) | 30 (33%) 62 (67%) | <.0001 |
BMI (mean ± SD) | 28.5 ± 4.4 | 28.1 ± 4.5 | .578 |
Abbreviations: BMI, body mass index; RCR, rotator cuff repair; rTSA, reverse total shoulder arthroplasty.
Table 2. Preoperative variables
| RCR (n=95) | rTSA (n=92) | P value |
Radiographic parameters | |||
AB interval | 9 ± 3 | 5 ± 3 | <.0001 |
Humeral escape | 14.1% | 50.6% | <.0001 |
Hamada 1 | 76.1% | 15.6% | <.0001 |
Hamada 2 | 13.0% | 50.6% | |
Hamada 3 | 10.9% | 33.8% | |
Goutallier grade 1 | 7.8% | 19.3% | .227 |
Goutallier grade 2 | 66.7% | 52.6% | |
Goutallier grade 3 | 21.6% | 19.3% | |
Goutallier grade 4 | 3.9% | 8.8% | |
Clinical measures | |||
Preop FE | 113 ± 50 | 57 ± 34 | <.0001 |
Preop AB | 97 ± 45 | 53 ± 35 | <.0001 |
Preop ER | 42 ± 25 | 32 ± 28 | .029 |
Preop IR | 2.9 ± 1.6 | 2.6 ± 1.8 | .247 |
Preop pain | 5.7 ± 2.3 | 5.6 ± 2.5 | .927 |
Preop ASES | 44 ± 17 | 39 ± 16 | .04 |
Preop SST | 3.1 ± 2.6 | 1.9 ± 1.7 | .001 |
Patients parameters | |||
Previous cuff surgery | 6.3% | 35.9% | <.0001 |
Comorbidity count | 1.7 ± 1.4 | 2.1 ± 2.7 | .126 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; preop, preoperative; SST, simple shoulder test.
Radiographically, patients selected to undergo rTSA had a smaller AHI (4.8 vs 8.7, P < .0001) and more evidence of superior subluxation (50.6% vs 14.1%, P < .0001) than those in the RCR group. Average Hamada grade was 1.4 ± 0.7 and 2.2 ± 0.7 for the RCR and rTSA groups, respectively (P < .0001). Average Goutallier grade was similar between the groups (2.2 ± 0.6 for RCR vs 2.2 ± 0.8 for rTSA, P =.227), and 25.5% of the RCR group had Grade 3 or 4 atrophy compared with 28.1% of the rTSA group.
POSTOPERATIVE OUTCOMES
The average follow-up time was 44 months for RCR and 47 months for rTSA. Patients in the RCR and rTSA groups were highly satisfied with the surgery (8.5 ± 2.6 vs 8.2 ± 2.6, P = .461) and had significantly increased range of motion in all planes and improved functional scores (Table 3). The rTSA group had greater net improvement in forward elevation, abduction, and external rotation than the RCR group. Both groups demonstrated similar improvement in ASES, SST, and VAS pain scores.
Table 3. Postoperative outcomes
| RCR (n=95) | P value | rTSA (n=92) | P value | ||
Preoperative | Postoperative | Preoperative | Postoperative | |||
FE | 113 ± 50 | 166 ± 26 | <.0001 | 57 ± 34 | 136 ± 46 | <.0001 |
AB | 97 ± 45 | 155 ± 37 | <.0001 | 53 ± 35 | 129 ± 44 | <.0001 |
ER | 42 ± 25 | 48 ± 20 | .033 | 32 ± 28 | 57 ± 32 | <.0001 |
IR | 2.9 ± 1.6 | 4.6 ± 1.6 | <.0001 | 2.6 ± 1.8 | 4.7 ± 2.4 | <.0001 |
VAS pain | 5.7 ± 2.3 | 1.7 ± 2.4 | <.0001 | 5.6 ± 2.5 | 1.6 ± 2.5 | <.0001 |
ASES | 44 ± 17 | 83 ± 18 | <.0001 | 39 ± 16 | 77 ± 22 | <.0001 |
SST | 3.1 ± 2.6 | 9.3 ± 2.9 | <.0001 | 1.9 ± 1.7 | 7.1 ± 3.4 | <.0001 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; SST, simple shoulder test; VAS – visual analog score.
In the RCR group, 5 patients (5.3%) required reoperation: 3 patients underwent conversion to rTSA, 1 patient underwent biceps tenotomy with subacromial decompression, and 1 patient underwent arthroscopic irrigation and debridement for a postoperative Propionibacterium acnes infection. In the rTSA group, 2 patients (2.2%) required reoperation: 1 patient underwent open reduction internal fixation for a scapula fracture that failed conservative management, and 1 patient had an open irrigation and debridement with polyethylene exchange for an acute postoperative infection of unknown source.
DISCUSSION
Massive, retracted rotator cuff tears are a common and difficult problem.1 The treatment options are numerous and depend on a variety of preoperative factors including patient-specific characteristics and factors specific to the tear. For certain patients, nonoperative management may be a reasonable first step, as an MCT does not necessarily preclude painless, functional shoulder motion. Elderly, lower demand individuals have been shown to do well with physical rehabilitation.18 Similarly, for the same category of elderly patients who do not respond to conservative measures, arthroscopic tendon debridement with or without subacromial decompression and/or biceps tenotomy may be effective.1,19 This technique has been described as “limited goals surgery;” despite some mixed results in the literature, multiple studies have reported symptomatic and functional improvement after simple debridement.2,19–21The consensus among several authors has been that this procedure continues to play a role for elderly, low-demand patients whose functional goals are limited and whose primary complaint is pain.1,2,20
For the majority of patients with MCT who desire pain relief and a restoration of shoulder function, RCR remains the gold standard of treatment and should be the primary aim if feasible. Complete RCR has consistently outperformed both partial repair and debridement in multiple studies in terms of pain relief and functional improvement.10,21,22However, elderly patients with chronic, massive tears, particularly in the setting of muscle atrophy, are at high risk of failure with attempted cuff repair.9,23 Novel techniques such as superior capsular reconstruction and subacromial balloon spacer implantation may offer a minimally invasive method of re-centering the humeral head and stabilizing the glenohumeral joint; however, these new treatment options lack any long-term data in the literature to support their widespread use.24–26 Alternatively, rTSA has been shown to be a reliable option to restore shoulder function in the setting of a massive irreparable rotator cuff tear, even in the absence of arthritis.5,27-31
Continue to: The decision-making process...
The decision-making process for selecting RCR or rTSA in the setting of MCT without arthritis in the older population (age >65 years) remains challenging. We attempted to quantify the data of a high-volume surgeon and identify the differences and similarities between those patients selected for either procedure. At our institution, we generally performed rTSA on patients with low preoperative range of motion, poor function based on SST and ASES scores, small AHI, and strong evidence of superior subluxation. We were also more likely to perform rTSA if the patient had a history of rotator cuff surgery. There was a predilection for older age and female gender in those who underwent rTSA.
For our study, we elected to focus on patients >65 years. In our experience, the choice of which surgical procedure to perform is generally easier in younger patients. Most surgeons appropriately opt for an arthroscopic procedure or tendon transfer to preserve bone and maintain the option of rTSA as a salvage procedure if necessary in the future. Studies have reported that <60 years is a predictor of poor outcome with rTSA, and patients <65 years who undergo rTSA have been shown to have high complication rates.30-32 Furthermore, the longevity of the implant in young patients is a significant concern, and revision surgery after rTSA is technically demanding and known to result in poor functional outcomes.32,33
Although the indications for rTSA are expanding, attempts at RCR in the setting of MCT remain largely appropriate. Preserved preoperative anterior elevation >90° has been associated with loss of motion after rTSA and poor satisfaction, and one should exercise caution when considering rTSA in this setting.3 The current study confirmed that even older patients with MCT may be very satisfied with arthroscopic RCR (Figure 1). Both range of motion and function significantly improved, and patients were largely satisfied with the procedure with an average self-reported outcome of good to excellent. At the time of final follow-up for this study, only 3 shoulders in the RCR group had undergone conversion to rTSA. This number may be expected to rise with long follow-up periods, and we feel that prolonging the time before arthroplasty is generally in the best interest of the patient.
Our results were consistent with several reported studies in which RCR has been shown to be successful in the setting of MCT.34–37 Henry and colleagues36 performed a systematic review that evaluated 954 patients who underwent partial or complete anatomic RCR for MCT. Although the average age was 63 years (range, 37–87), functional outcome scores, VAS pain score, and overall range of motion consistently and significantly improved.
rTSA may be a “more reliable” option than RCR in treating MCT in the older population because it does not rely on tendon healing. However, the relationship between tendon healing and clinical outcomes after RCR is unclear. The aforementioned systematic review reported re-tear rates to be as high as 79%, but several studies have reported high satisfaction even in the setting of retear.36 Yoo and colleagues38 and Chung and colleagues9 reported re-tear rates of 45.5% and 39.8%, respectively, but both studies noted that there was no difference in outcome measures between those patients with and without re-tears. In particular, for patients who have had no prior rotator cuff surgery, an attempt at arthroscopic repair may be a prudent option with relatively low risk.
Although certain patients may clinically improve despite suffering a re-tear (or inability to heal in the first place), others continue to experience pain and dysfunction that negatively affect their quality of life.39–41 These patients are more often appropriate candidates for rTSA. Indeed, several studies have demonstrated a higher re-tear rate in patients with a history of surgery than in those without.23,31,38,42 Shamsudin and colleagues43 found revision arthroscopic RCR, even in a younger age group with tears of all sizes, to be twice as likely to re-tear. Notably, re-tear after revision repair may be more likely to be symptomatic, as these re-tears are routinely associated with pain, stiffness, and loss of function. Even in the hands of experienced surgeons in a younger population, revision repair has only been able to reverse pseudoparalysis in 43% of patients, leading to only 39% return to sport or full activity.44 In examining our data, we were much less likely to perform an RCR in patients who had a history of cuff repair surgery than in those without this history.
Continue to: Overall, those patients selected for rTSA...
Overall, those patients selected for rTSA in our study population performed well postoperatively (Figure 2 and Figure 3). Vast improvements were noted in range of motion, function, and pain scores at final follow up. Moreover, no patients in the study group required revision arthroplasty during the follow-up period. Although the average follow-up period was only 47 months, these results suggested that elderly patients with MCT without arthritis may be particularly ideal candidates for rTSA with regard to implant survival and anticipated revision rate when chosen appropriately.
Several weaknesses were noted within this paper. First, the study was retrospective, precluding randomization of treatment groups and standardization of data collection and follow-up. The outcomes of RCR and rTSA could not be compared directly due to the inherent selection bias. The groups clearly differed in many respects, and these preoperative factors likely played a role in postoperative outcomes. However, the primary goal of this study was not to compare outcomes of the treatment groups but to analyze the patterns of patient selection by an experienced treating surgeon and contribute to published data that each surgery can be successful in this patient population when chosen appropriately.
Second, our data were based on a single surgeon’s decisions, and results may not be generalizable. Furthermore, the senior author has had a longstanding interest in reverse shoulder arthroplasty and has published data illustrating successful outcomes for rTSA in patients with MCT. For this reason, one could presume that there may have been some bias toward treating patients with rTSA. However, we feel that the senior author’s unique and longstanding experience in treating MCT allows for a thorough evaluation and comparison of preoperative variables and outcomes declared within this study. Indeed, many patients included in this study were referred from outside institutions specifically for rTSA but instead were deemed more appropriate candidates for RCR and underwent successful arthroscopic repair, a common scenario which served as an impetus for this study.
CONCLUSION
RCR and rTSA are both viable options for patients >65 years with MCT without arthritis. Treatment must be individualized for each patient with careful consideration of a number of preoperative variables and patient characteristics. At our institution, patients with previous RCR, decreased range of motion, poor function, and strong radiographic evidence of subluxation are more likely to undergo rTSA. When chosen appropriately, both RCR and rTSA can result in improved range of motion, function, and high patient satisfaction in this patient population.
ABSTRACT
The decision to perform rotator cuff repair (RCR) versus reverse total shoulder arthroplasty (rTSA) for massive rotator cuff tear (MCT) without arthritis can be difficult. Our aim was to identify preoperative variables that are influential in a surgeon's decision to choose one of the two procedures and evaluate outcomes.
We retrospectively reviewed 181 patients older than 65 who underwent RCR or rTSA for MCT without arthritis. Clinical and radiographic data were collected and used to evaluate the preoperative variables in each of these two patient populations and assess outcomes.
Ninety-five shoulders underwent RCR and 92 underwent rTSA with an average followup of 44 and 47 months, respectively. Patients selected for RCR had greater preoperative flexion (113 vs 57), abduction (97 vs 53), and external rotation (42 vs 32), higher SST (3.1 vs 1.9) and ASES scores (43.8 vs 38.6), and were less likely to have had previous cuff surgery (6.3% vs 35.9%). Patients selected for rTSA had a smaller acromiohumeral interval (4.8 vs 8.7) and more superior subluxation (50.6% vs 14.1%). Similar preoperative characteristics included pain, comorbidities, and BMI. Patients were satisfied in both groups and had significant improvement in motion and function postoperatively.
Both RCR and rTSA can result in significant functional improvement and patient satisfaction in the setting of MCT without arthritis in patients older than 65. At our institution, patients who underwent rTSA had less pre-operative motion, lower function, more evidence of superior migration, and were more likely to have had previous rotator cuff surgery.
Continue to: The treatment of patients...
The treatment of patients with massive rotator cuff tears (MCTs) without osteoarthritis is challenging. This population is of considerable interest, as the prevalence of MCT has been reported to be as high as 40% of all rotator cuff tears.1Options for surgical treatment in patients who have failed conservative management are numerous and include tendon debridement, partial or complete arthroscopic or open rotator cuff repair (RCR), tendon transfers, reverse total shoulder arthroplasty (rTSA), arthroscopic superior capsular reconstruction (ASCR), and other grafting procedures.2 Arthroscopic superior capsular reconstruction shows promise as a novel technique, but it is not yet well studied. Other procedures such as tendon transfers fit into the treatment algorithm for only a small subset of patients. Open rotator cuff repair and rTSA are the 2 most commonly utilized procedures for MCT, and both have been shown to reliably achieve significant functional improvement and patient satisfaction.3–6
The dilemma for the treating surgeon is deciding which patients to treat with RCR and who to treat with rTSA. Predicting which surgical procedure will provide a better functional result is difficult and controversial.7 The RCR method is a bone-conserving procedure with relatively low surgical risk and allows the option for rTSA to be performed as a salvage surgery should repair fail. It also may be less costly in the appropriate population.8 However, large rotator cuff tears in elderly patients have low healing potential, and the prospect of participating in a lengthy rehabilitation after an operation that may not prove successful can be deterring.9,10 In the elderly population, rTSA may be a reliable option, as tendon healing of the cuff is not necessary to restore function. However, rTSA does not conserve bone, provides a non-anatomic solution, and has had historically high complication rates.4,5
In an effort to aid in the decision-making process when considering these 2 surgical options, we compared RCR and rTSA performed at a single institution for MCT in patients >65 years. Our aim was to identify preoperative patient variables that influence a surgeon’s decision to proceed with 1 of the 2 procedures. Moreover, we evaluated clinical outcomes in these 2 patient populations. We hypothesized that (1) patients selected for rTSA would have worse preoperative function, less range of motion, more comorbidities, more evidence of radiographic subluxation, and a higher likelihood of having undergone previous RCR than those selected for RCR, and (2) both RCR and rTSA would be successful and result in improved clinical outcomes with high patient satisfaction.
MATERIALS AND METHODS
PATIENT SELECTION
We performed a retrospective chart review using our practice database of all patients undergoing arthroscopic RCR and rTSA for any indication by the senior author (M.A.F.) between January 2004 and April 2015. A total of 1503 RCRs and 1973 rTSAs were conducted during the study period. Patient medical records were reviewed, and those meeting the following criteria were included in the study: >65 years at the time of surgery, MCT, no preoperative glenohumeral arthritis, minimum follow-up of 12 months, functional deltoid muscle on physical examination, and no prior shoulder surgery except for RCR or diagnostic arthroscopy. A total of 92 patients who underwent arthroscopic RCR and 89 patients who underwent rTSA met the inclusion criteria. For patients with bilateral shoulder surgery, we measured each shoulder independently. Three patients underwent bilateral rTSA, and 3 patients underwent bilateral RCR, leaving 95 shoulders in the RCR group and 92 in the rTSA group. The Western Institutional Review Board determined this study to be exempt from review.
RADIOGRAPHIC EVALUATION
All patient charts included a radiology report and documented interpretation of the images by the treating surgeon prior to surgery. Radiographs were assessed to assure the absence of preoperative glenohumeral osteoarthritis. The images were also graded based on the Hamada classification.11 Stage 1 is associated with minimal radiographic change with an acromiohumeral interval (AHI) >6 mm; stage 2 is characterized by narrowing of the AHI <6 mm; and Stage 3 is defined by narrowing of the AHI with radiographic changes of the acromion. Stages 4 and higher include arthritic changes to the glenohumeral joint, and they were not included in the study population. The AHI measurements and the presence or absence of glenohumeral subluxation were documented.
Continue to: MASSIVE CUFF TEAR DETERMINATION...
MASSIVE CUFF TEAR DETERMINATION
We defined MCT on the basis of previously described criteria of tears involving ≥2 tendons or tears measuring ≥5 cm in greatest dimension.12,13 Patient charts were screened, and those whose clinical notes or radiology reports indicated an absence of MCT were excluded. Preoperative imaging of the remaining patients was then evaluated by 3 fellowship-trained shoulder surgeons to confirm MCT in all patients with a clinically documented MCT, as well as to assess those who had insufficient documentation of tear size in the notes.
Advanced imaging was evaluated for fatty atrophy of the rotator cuff musculature, and Goutallier classification was assigned.14,15 Length of retraction was measured from the tendon end to the medial aspect of the footprint on coronal imaging, and the subscapularis and teres minor were assessed and documented as torn or intact.16,17
DATA COLLECTION
We reviewed clinical charts and patient questionnaire forms from both the preoperative and follow-up visits. Clinical data collected included gender, age at surgery, active range of motion (forward elevation, abduction, external rotation, and internal rotation), comorbidities, smoking status, BMI, history of shoulder surgery, and any postoperative complications or need for secondary surgery. All patients completed patient-centered questionnaires regarding shoulder pain and dysfunction at each visit or via telephone communication with clinic staff. Outcome measurements used for analysis included American Shoulder and Elbow Surgeons (ASES) Score, simple shoulder test (SST), visual analog score (VAS) pain scale, and patient-reported satisfaction (Graded 1-10; 1 = poor outcome; 4 = satisfactory outcome; 7 = good outcome; 10 = excellent outcome).
DATA ANALYSIS AND STATISTICAL METHODS
Statistical tests were selected based on the result of Shapiro–Wilk test for normality. Continuous variables were evaluated with either independent t test or Mann–Whitney U test. Dependent t test was used to evaluate outcome variables. For categorical variables, either Pearson’s χ2or Fisher’s exact test was performed depending on the sample size. Alpha was set at P =.05.
Continue to: RESULTS...
RESULTS
PREOPERATIVE CHARACTERISTICS
Of the 187 shoulders in the study group, 95 had RCR and 92 had rTSA. Demographic information and preoperative variables for both groups are summarized in Table 1 and Table 2. Patients in the RCR group had greater preoperative forward elevation, abduction, and external rotation and higher preoperative functional scores than those in the rTSA group. Patients in the rTSA group were older and more likely to be female than those in the RCR group. More patients in the rTSA group had undergone prior RCR compared with those in the RCR group. Each of these differences was statistically significant. Subjective pain scores, BMI, and comorbidities were similar between the 2 groups.
Table 1. Patient demographics
| RCR | rTSA | P value |
Age (yr; mean ± SD) | 71 ± 5 | 74 ± 6 | <.0001 |
Gender *male (no.; %) *female (no.; %) | 57 (60%) 38 (40%) | 30 (33%) 62 (67%) | <.0001 |
BMI (mean ± SD) | 28.5 ± 4.4 | 28.1 ± 4.5 | .578 |
Abbreviations: BMI, body mass index; RCR, rotator cuff repair; rTSA, reverse total shoulder arthroplasty.
Table 2. Preoperative variables
| RCR (n=95) | rTSA (n=92) | P value |
Radiographic parameters | |||
AB interval | 9 ± 3 | 5 ± 3 | <.0001 |
Humeral escape | 14.1% | 50.6% | <.0001 |
Hamada 1 | 76.1% | 15.6% | <.0001 |
Hamada 2 | 13.0% | 50.6% | |
Hamada 3 | 10.9% | 33.8% | |
Goutallier grade 1 | 7.8% | 19.3% | .227 |
Goutallier grade 2 | 66.7% | 52.6% | |
Goutallier grade 3 | 21.6% | 19.3% | |
Goutallier grade 4 | 3.9% | 8.8% | |
Clinical measures | |||
Preop FE | 113 ± 50 | 57 ± 34 | <.0001 |
Preop AB | 97 ± 45 | 53 ± 35 | <.0001 |
Preop ER | 42 ± 25 | 32 ± 28 | .029 |
Preop IR | 2.9 ± 1.6 | 2.6 ± 1.8 | .247 |
Preop pain | 5.7 ± 2.3 | 5.6 ± 2.5 | .927 |
Preop ASES | 44 ± 17 | 39 ± 16 | .04 |
Preop SST | 3.1 ± 2.6 | 1.9 ± 1.7 | .001 |
Patients parameters | |||
Previous cuff surgery | 6.3% | 35.9% | <.0001 |
Comorbidity count | 1.7 ± 1.4 | 2.1 ± 2.7 | .126 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; preop, preoperative; SST, simple shoulder test.
Radiographically, patients selected to undergo rTSA had a smaller AHI (4.8 vs 8.7, P < .0001) and more evidence of superior subluxation (50.6% vs 14.1%, P < .0001) than those in the RCR group. Average Hamada grade was 1.4 ± 0.7 and 2.2 ± 0.7 for the RCR and rTSA groups, respectively (P < .0001). Average Goutallier grade was similar between the groups (2.2 ± 0.6 for RCR vs 2.2 ± 0.8 for rTSA, P =.227), and 25.5% of the RCR group had Grade 3 or 4 atrophy compared with 28.1% of the rTSA group.
POSTOPERATIVE OUTCOMES
The average follow-up time was 44 months for RCR and 47 months for rTSA. Patients in the RCR and rTSA groups were highly satisfied with the surgery (8.5 ± 2.6 vs 8.2 ± 2.6, P = .461) and had significantly increased range of motion in all planes and improved functional scores (Table 3). The rTSA group had greater net improvement in forward elevation, abduction, and external rotation than the RCR group. Both groups demonstrated similar improvement in ASES, SST, and VAS pain scores.
Table 3. Postoperative outcomes
| RCR (n=95) | P value | rTSA (n=92) | P value | ||
Preoperative | Postoperative | Preoperative | Postoperative | |||
FE | 113 ± 50 | 166 ± 26 | <.0001 | 57 ± 34 | 136 ± 46 | <.0001 |
AB | 97 ± 45 | 155 ± 37 | <.0001 | 53 ± 35 | 129 ± 44 | <.0001 |
ER | 42 ± 25 | 48 ± 20 | .033 | 32 ± 28 | 57 ± 32 | <.0001 |
IR | 2.9 ± 1.6 | 4.6 ± 1.6 | <.0001 | 2.6 ± 1.8 | 4.7 ± 2.4 | <.0001 |
VAS pain | 5.7 ± 2.3 | 1.7 ± 2.4 | <.0001 | 5.6 ± 2.5 | 1.6 ± 2.5 | <.0001 |
ASES | 44 ± 17 | 83 ± 18 | <.0001 | 39 ± 16 | 77 ± 22 | <.0001 |
SST | 3.1 ± 2.6 | 9.3 ± 2.9 | <.0001 | 1.9 ± 1.7 | 7.1 ± 3.4 | <.0001 |
Abbreviations: AB, abduction; ASES, American Shoulder and Elbow Society score; ER, external rotation; FE, forward elevation; IR, internal rotation; SST, simple shoulder test; VAS – visual analog score.
In the RCR group, 5 patients (5.3%) required reoperation: 3 patients underwent conversion to rTSA, 1 patient underwent biceps tenotomy with subacromial decompression, and 1 patient underwent arthroscopic irrigation and debridement for a postoperative Propionibacterium acnes infection. In the rTSA group, 2 patients (2.2%) required reoperation: 1 patient underwent open reduction internal fixation for a scapula fracture that failed conservative management, and 1 patient had an open irrigation and debridement with polyethylene exchange for an acute postoperative infection of unknown source.
DISCUSSION
Massive, retracted rotator cuff tears are a common and difficult problem.1 The treatment options are numerous and depend on a variety of preoperative factors including patient-specific characteristics and factors specific to the tear. For certain patients, nonoperative management may be a reasonable first step, as an MCT does not necessarily preclude painless, functional shoulder motion. Elderly, lower demand individuals have been shown to do well with physical rehabilitation.18 Similarly, for the same category of elderly patients who do not respond to conservative measures, arthroscopic tendon debridement with or without subacromial decompression and/or biceps tenotomy may be effective.1,19 This technique has been described as “limited goals surgery;” despite some mixed results in the literature, multiple studies have reported symptomatic and functional improvement after simple debridement.2,19–21The consensus among several authors has been that this procedure continues to play a role for elderly, low-demand patients whose functional goals are limited and whose primary complaint is pain.1,2,20
For the majority of patients with MCT who desire pain relief and a restoration of shoulder function, RCR remains the gold standard of treatment and should be the primary aim if feasible. Complete RCR has consistently outperformed both partial repair and debridement in multiple studies in terms of pain relief and functional improvement.10,21,22However, elderly patients with chronic, massive tears, particularly in the setting of muscle atrophy, are at high risk of failure with attempted cuff repair.9,23 Novel techniques such as superior capsular reconstruction and subacromial balloon spacer implantation may offer a minimally invasive method of re-centering the humeral head and stabilizing the glenohumeral joint; however, these new treatment options lack any long-term data in the literature to support their widespread use.24–26 Alternatively, rTSA has been shown to be a reliable option to restore shoulder function in the setting of a massive irreparable rotator cuff tear, even in the absence of arthritis.5,27-31
Continue to: The decision-making process...
The decision-making process for selecting RCR or rTSA in the setting of MCT without arthritis in the older population (age >65 years) remains challenging. We attempted to quantify the data of a high-volume surgeon and identify the differences and similarities between those patients selected for either procedure. At our institution, we generally performed rTSA on patients with low preoperative range of motion, poor function based on SST and ASES scores, small AHI, and strong evidence of superior subluxation. We were also more likely to perform rTSA if the patient had a history of rotator cuff surgery. There was a predilection for older age and female gender in those who underwent rTSA.
For our study, we elected to focus on patients >65 years. In our experience, the choice of which surgical procedure to perform is generally easier in younger patients. Most surgeons appropriately opt for an arthroscopic procedure or tendon transfer to preserve bone and maintain the option of rTSA as a salvage procedure if necessary in the future. Studies have reported that <60 years is a predictor of poor outcome with rTSA, and patients <65 years who undergo rTSA have been shown to have high complication rates.30-32 Furthermore, the longevity of the implant in young patients is a significant concern, and revision surgery after rTSA is technically demanding and known to result in poor functional outcomes.32,33
Although the indications for rTSA are expanding, attempts at RCR in the setting of MCT remain largely appropriate. Preserved preoperative anterior elevation >90° has been associated with loss of motion after rTSA and poor satisfaction, and one should exercise caution when considering rTSA in this setting.3 The current study confirmed that even older patients with MCT may be very satisfied with arthroscopic RCR (Figure 1). Both range of motion and function significantly improved, and patients were largely satisfied with the procedure with an average self-reported outcome of good to excellent. At the time of final follow-up for this study, only 3 shoulders in the RCR group had undergone conversion to rTSA. This number may be expected to rise with long follow-up periods, and we feel that prolonging the time before arthroplasty is generally in the best interest of the patient.
Our results were consistent with several reported studies in which RCR has been shown to be successful in the setting of MCT.34–37 Henry and colleagues36 performed a systematic review that evaluated 954 patients who underwent partial or complete anatomic RCR for MCT. Although the average age was 63 years (range, 37–87), functional outcome scores, VAS pain score, and overall range of motion consistently and significantly improved.
rTSA may be a “more reliable” option than RCR in treating MCT in the older population because it does not rely on tendon healing. However, the relationship between tendon healing and clinical outcomes after RCR is unclear. The aforementioned systematic review reported re-tear rates to be as high as 79%, but several studies have reported high satisfaction even in the setting of retear.36 Yoo and colleagues38 and Chung and colleagues9 reported re-tear rates of 45.5% and 39.8%, respectively, but both studies noted that there was no difference in outcome measures between those patients with and without re-tears. In particular, for patients who have had no prior rotator cuff surgery, an attempt at arthroscopic repair may be a prudent option with relatively low risk.
Although certain patients may clinically improve despite suffering a re-tear (or inability to heal in the first place), others continue to experience pain and dysfunction that negatively affect their quality of life.39–41 These patients are more often appropriate candidates for rTSA. Indeed, several studies have demonstrated a higher re-tear rate in patients with a history of surgery than in those without.23,31,38,42 Shamsudin and colleagues43 found revision arthroscopic RCR, even in a younger age group with tears of all sizes, to be twice as likely to re-tear. Notably, re-tear after revision repair may be more likely to be symptomatic, as these re-tears are routinely associated with pain, stiffness, and loss of function. Even in the hands of experienced surgeons in a younger population, revision repair has only been able to reverse pseudoparalysis in 43% of patients, leading to only 39% return to sport or full activity.44 In examining our data, we were much less likely to perform an RCR in patients who had a history of cuff repair surgery than in those without this history.
Continue to: Overall, those patients selected for rTSA...
Overall, those patients selected for rTSA in our study population performed well postoperatively (Figure 2 and Figure 3). Vast improvements were noted in range of motion, function, and pain scores at final follow up. Moreover, no patients in the study group required revision arthroplasty during the follow-up period. Although the average follow-up period was only 47 months, these results suggested that elderly patients with MCT without arthritis may be particularly ideal candidates for rTSA with regard to implant survival and anticipated revision rate when chosen appropriately.
Several weaknesses were noted within this paper. First, the study was retrospective, precluding randomization of treatment groups and standardization of data collection and follow-up. The outcomes of RCR and rTSA could not be compared directly due to the inherent selection bias. The groups clearly differed in many respects, and these preoperative factors likely played a role in postoperative outcomes. However, the primary goal of this study was not to compare outcomes of the treatment groups but to analyze the patterns of patient selection by an experienced treating surgeon and contribute to published data that each surgery can be successful in this patient population when chosen appropriately.
Second, our data were based on a single surgeon’s decisions, and results may not be generalizable. Furthermore, the senior author has had a longstanding interest in reverse shoulder arthroplasty and has published data illustrating successful outcomes for rTSA in patients with MCT. For this reason, one could presume that there may have been some bias toward treating patients with rTSA. However, we feel that the senior author’s unique and longstanding experience in treating MCT allows for a thorough evaluation and comparison of preoperative variables and outcomes declared within this study. Indeed, many patients included in this study were referred from outside institutions specifically for rTSA but instead were deemed more appropriate candidates for RCR and underwent successful arthroscopic repair, a common scenario which served as an impetus for this study.
CONCLUSION
RCR and rTSA are both viable options for patients >65 years with MCT without arthritis. Treatment must be individualized for each patient with careful consideration of a number of preoperative variables and patient characteristics. At our institution, patients with previous RCR, decreased range of motion, poor function, and strong radiographic evidence of subluxation are more likely to undergo rTSA. When chosen appropriately, both RCR and rTSA can result in improved range of motion, function, and high patient satisfaction in this patient population.
- Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894-1908. doi:10.2106/JBJS.I.01531.
- Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493-1505. doi:10.1016/j.jse.2015.04.005.
- Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600-606. doi:10.1016/j.jse.2009.03.011.
- Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244-1251. doi:10.2106/JBJS.G.00775.
- Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544-2556.doi:10.2106/JBJS.I.00912.
- Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476-1485. doi:10.2106/JBJS.F.00666.
- Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
- Makhni EC, Swart E, Steinhaus ME, Mather RC 3rd, Levine WN, Bach BR Jr et al. Cost-effectiveness of reverse total shoulder arthroplasty versus arthroscopic rotator cuff repair for symptomatic large and massive rotator cuff tears. Arthroscopy. 2016;32(9):1771-1780. doi:10.1016/j.arthro.2016.01.063.
- Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41:1674-1683. doi:10.1177/0363546513485719.
- Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180. doi:10.1186/1471-2474-15-180.
- Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
- DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563-567.
- Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505-515.
- Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599-605.
- Goutallier D, Bernageau J, Patte D. Assessment of the trophicity of the muscles of the ruptured rotator cuff by CT scan. In: Post M, Morrey B, Hawkins R, eds. Surgery of the Shoulder. St. Louis, MO: Mosby, 1990;11-13.
- Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
- Meyer DC, Wieser K, Farshad M, Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40:2242-2247.
- Williams GR Jr, Rockwood CA Jr, Bigliani LU, Ianotti JP, Stanwood W. Rotator cuff tears: why do we repair them? J Bone Joint Surg Am. 2004;86-A(12):2764-2776.
- Rockwood CA Jr, Williams GR Jr, Burkhead WZ Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857-866.
- Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthopaed Traumatol. 2010;11:13-20. doi 10.1007/s10195-010-0084-0.
- Heuberer PR, Kolblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24:3828-3837.
- Moser M, Jablonski MV, Horodyski M, Wright TW. Functional outcome of surgically treated massive rotator cuff tears: a comparison of complete repair, partial repair, and debridement. Orthopedics.2007;30(6):479-482.
- Rhee YG, Cho NS, Yoo JH. Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy. 2014;30:546-554. doi:10.1016/j.arthro.2014.02.006.
- Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93-99. doi: 10.1016/j.arthro.2017.08.265.
- Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy.2013;29:459-70.
- Piekaar RSM, Bouman ICE, van Kampen PM, van Eijk F, Huijsmans PE. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskelet Surg. 2018;102(3):247-255. doi: 10.1007/s12306-017-0525-5.
- Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23:1662-1668. doi:10.1016/j.jse.2014.03.001.
- Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S-161S. doi:10.1016/j.jse.2004.10.006.
- Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-68. doi: 10.3928/0147-7447-19930101-11.
- Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24:1698-1706. doi:10.1016/j.jse.2015.04.015.
- Kim HM, Caldwell JM, Buza JA, Fink LA, Ahmad CS, Bigliani LU, Levine WN. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96:106-112. doi:10.2106/JBJS.L.01649.
- Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208. doi:10.1016/j.jse.2012.11.016.
- Sershon RA, Van Thiel GS, Lin EC, McGill KC, Cole BJ, Verma NN, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg.2014;23:395-400. doi:10.1016/j.jse.2013.07.047.
- Denard PJ, Ladermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28:1214-1219. doi:10.1016/j.arthro.2012.02.026.
- Denard PJ, Ladermann A, Brady PC, Narbona P, Adams CR, Arrigoni P, et al. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med. 2015;43:2373-2378. doi: 10.1177/0363546515597486.
- Henry P, Wasserstein D, Park S, Dwyer T, Chahal J, Slobogean G, Schemitsch E. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31:2472-2480. doi:10.1016/j.arthro.2015.06.038.
- Oh JH, Kim SH, Shin SH, Chung SW, Kim JY, Kim SJ. Outcome of rotator cuff repair in large-to-massive tear with pseudoparalysis: a comparative study with propensity score matching. Am J Sports Med.2011;39:1413-1420.
- Yoo JC, Ahn JH, Koh KH, Lim KS. Rotator cuff integrity after arthroscopic repair for large tears with less-than-optimal footprint coverage. Arthroscopy. 2009;25:1093-1100. doi:10.1016/j.arthro.2009.07.010.
- Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304-314.
- Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, Flatow EL Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. Am J Sports Med. 2004;32:1716-1722.
- Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10:54-60.
- Papadopoulos P, Karataglis D, Boutsiadis A, Fotiadou A, Christoforidis J, Christodoulou A. Functional outcome and structural integrity following mini-open repair of large and massive rotator cuff tears: a 3-5 year follow-up study. J Shoulder Elbow Surg. 2011;20:131-137. doi:10.1016/j.jse.2010.05.026.
- Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med.2015;43:557-564. doi:10.1177/0363546514560729.
- Ladermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy. 2011;27:1620-1627. doi:10.1016/j.arthro.2011.08.290.
- Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894-1908. doi:10.2106/JBJS.I.01531.
- Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493-1505. doi:10.1016/j.jse.2015.04.005.
- Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600-606. doi:10.1016/j.jse.2009.03.011.
- Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244-1251. doi:10.2106/JBJS.G.00775.
- Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544-2556.doi:10.2106/JBJS.I.00912.
- Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476-1485. doi:10.2106/JBJS.F.00666.
- Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113-120.
- Makhni EC, Swart E, Steinhaus ME, Mather RC 3rd, Levine WN, Bach BR Jr et al. Cost-effectiveness of reverse total shoulder arthroplasty versus arthroscopic rotator cuff repair for symptomatic large and massive rotator cuff tears. Arthroscopy. 2016;32(9):1771-1780. doi:10.1016/j.arthro.2016.01.063.
- Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41:1674-1683. doi:10.1177/0363546513485719.
- Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180. doi:10.1186/1471-2474-15-180.
- Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
- DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563-567.
- Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505-515.
- Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599-605.
- Goutallier D, Bernageau J, Patte D. Assessment of the trophicity of the muscles of the ruptured rotator cuff by CT scan. In: Post M, Morrey B, Hawkins R, eds. Surgery of the Shoulder. St. Louis, MO: Mosby, 1990;11-13.
- Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
- Meyer DC, Wieser K, Farshad M, Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40:2242-2247.
- Williams GR Jr, Rockwood CA Jr, Bigliani LU, Ianotti JP, Stanwood W. Rotator cuff tears: why do we repair them? J Bone Joint Surg Am. 2004;86-A(12):2764-2776.
- Rockwood CA Jr, Williams GR Jr, Burkhead WZ Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857-866.
- Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthopaed Traumatol. 2010;11:13-20. doi 10.1007/s10195-010-0084-0.
- Heuberer PR, Kolblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24:3828-3837.
- Moser M, Jablonski MV, Horodyski M, Wright TW. Functional outcome of surgically treated massive rotator cuff tears: a comparison of complete repair, partial repair, and debridement. Orthopedics.2007;30(6):479-482.
- Rhee YG, Cho NS, Yoo JH. Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy. 2014;30:546-554. doi:10.1016/j.arthro.2014.02.006.
- Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34(1):93-99. doi: 10.1016/j.arthro.2017.08.265.
- Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy.2013;29:459-70.
- Piekaar RSM, Bouman ICE, van Kampen PM, van Eijk F, Huijsmans PE. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskelet Surg. 2018;102(3):247-255. doi: 10.1007/s12306-017-0525-5.
- Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23:1662-1668. doi:10.1016/j.jse.2014.03.001.
- Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S-161S. doi:10.1016/j.jse.2004.10.006.
- Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-68. doi: 10.3928/0147-7447-19930101-11.
- Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24:1698-1706. doi:10.1016/j.jse.2015.04.015.
- Kim HM, Caldwell JM, Buza JA, Fink LA, Ahmad CS, Bigliani LU, Levine WN. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96:106-112. doi:10.2106/JBJS.L.01649.
- Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208. doi:10.1016/j.jse.2012.11.016.
- Sershon RA, Van Thiel GS, Lin EC, McGill KC, Cole BJ, Verma NN, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg.2014;23:395-400. doi:10.1016/j.jse.2013.07.047.
- Denard PJ, Ladermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28:1214-1219. doi:10.1016/j.arthro.2012.02.026.
- Denard PJ, Ladermann A, Brady PC, Narbona P, Adams CR, Arrigoni P, et al. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med. 2015;43:2373-2378. doi: 10.1177/0363546515597486.
- Henry P, Wasserstein D, Park S, Dwyer T, Chahal J, Slobogean G, Schemitsch E. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31:2472-2480. doi:10.1016/j.arthro.2015.06.038.
- Oh JH, Kim SH, Shin SH, Chung SW, Kim JY, Kim SJ. Outcome of rotator cuff repair in large-to-massive tear with pseudoparalysis: a comparative study with propensity score matching. Am J Sports Med.2011;39:1413-1420.
- Yoo JC, Ahn JH, Koh KH, Lim KS. Rotator cuff integrity after arthroscopic repair for large tears with less-than-optimal footprint coverage. Arthroscopy. 2009;25:1093-1100. doi:10.1016/j.arthro.2009.07.010.
- Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304-314.
- Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, Flatow EL Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. Am J Sports Med. 2004;32:1716-1722.
- Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10:54-60.
- Papadopoulos P, Karataglis D, Boutsiadis A, Fotiadou A, Christoforidis J, Christodoulou A. Functional outcome and structural integrity following mini-open repair of large and massive rotator cuff tears: a 3-5 year follow-up study. J Shoulder Elbow Surg. 2011;20:131-137. doi:10.1016/j.jse.2010.05.026.
- Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med.2015;43:557-564. doi:10.1177/0363546514560729.
- Ladermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy. 2011;27:1620-1627. doi:10.1016/j.arthro.2011.08.290.
TAKE-HOME POINTS
- Rotator cuff repair and reverse total shoulder arthroplasty are both viable options for patients >65 years with massive rotator cuff tears without arthritis.
- Treatment must be individualized for each patient, with careful consideration of a number of preoperative variables and patient characteristics.
- At our institution, patients with previous rotator cuff repair, decreased range of motion, poor function, and strong radiographic evidence of subluxation were more likely to undergo reverse total shoulder arthroplasty.
- Patients selected for rotator cuff repair had greater preoperative flexion, abduction, and external rotation, as well as higher functional scores, and were less likely to have had previous cuff surgery.
- When chosen appropriately, both rotator cuff repair and reverse total shoulder arthroplasty can result in improved range of motion, function, and high patient satisfaction in this patient population.
Arthroscopic SLAP IIb Repair Using Knot-Tying Versus Knotless Suture Anchors: Is There a Difference?
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
TAKE-HOME POINTS
- SLAP IIb tears are common injuries in overhead athletes, yet surgical outcomes are variable, with throwers commonly having difficulty with return to play at the same level.
- In this study, 92% of athletes returned to play post-operatively, yet only around 55% returned at the same level.
- In overhead athletes, overall return to play was 85.7%, yet only 39.3% returned at the same level.
- Knotless fixation required less revision surgery and had higher outcome scores and return to play when compared to knotted fixation; however, this did not reach statistical significance.
- Knotless fixation should be considered in SLAP IIb repairs given their lower profile leading to less rotator cuff irritation, the ability to better provide more consistent tensioning, and decreased surgical time.
Ultrasound excels for assessing shoulder dislocation
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
REPORTING FROM ACEP18
Key clinical point: Point-of-care ultrasound is accurate, simple, and fast, relative to x-ray, for the evaluation of shoulder dislocation.
Major finding: Based on results from 42 patients, time from triage to ultrasound, which had a sensitivity of 96% and specificity of 100%, was 60 minutes versus 100 minutes for x-ray.
Study details: An ongoing prospective, multicenter, observational study.
Disclosures: Dr. Secko reported no financial relationships relevant to this study.
The PASTA Bridge – A Repair Technique for Partial Articular-Sided Rotator Cuff Tears: A Biomechanical Evaluation of Construct Strength
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.
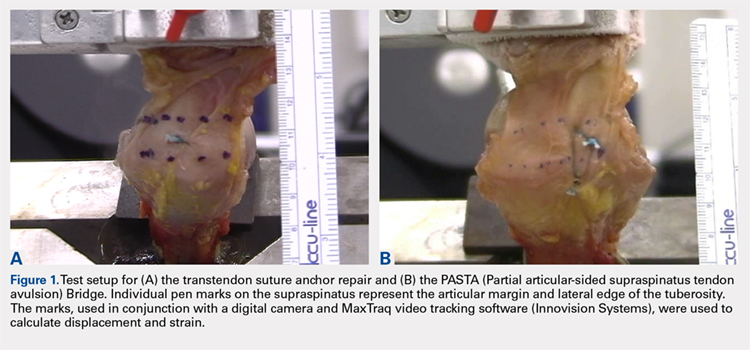
Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.
Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.
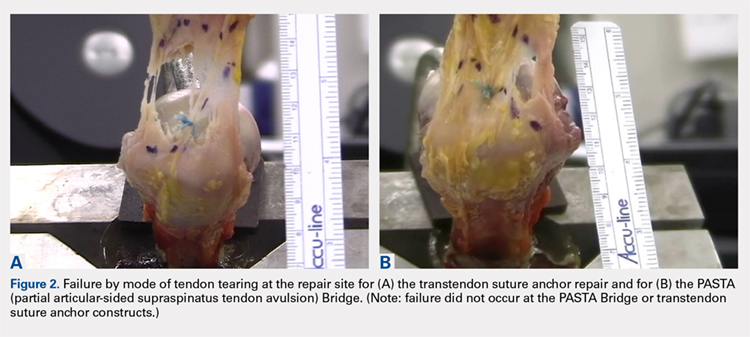
For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.
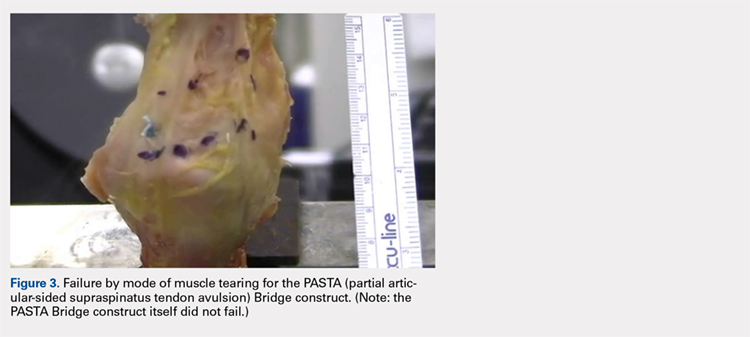
RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.
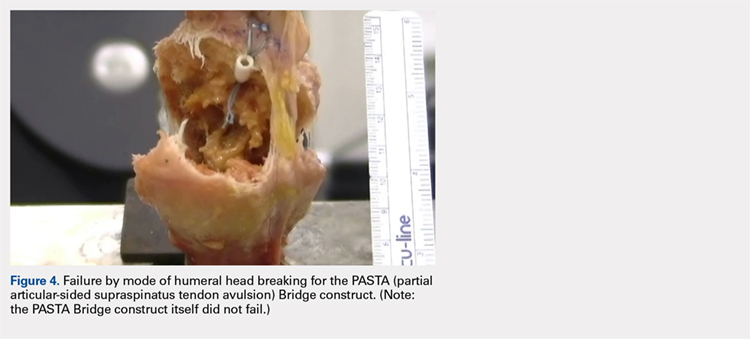
Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.

Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.

Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.

For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.

RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.

Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.

Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.

Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.

For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.

RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.

Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
TAKE-HOME POINTS
- The PASTA Bridge is biomechanically equivalent to the gold-standard transtendon repair technique.
- The configuration is a double-row repair, increasing the number of fixation points.
- The lateral anchor of the PASTA Bridge assumes the stress of the repair, allowing the medial anchors to act as pivot points.
- The PASTA Bridge is strong and capable of withstanding excessive forces.
- The PASTA Bridge poses less risk of complication.
Upper Extremity Injuries in Soccer
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
TAKE-HOME POINTS
- Upper extremity injuries in soccer are not common, however they can reach up to 18% of all injuries in professional goalkeepers.
- Common injury locations in the upper extremity in soccer are the shoulder/clavicle, hand/finger/thumb, the elbow, and the wrist and most of these injuries are traumatic injuries.
- Mechanism of injury, players’ complaints and presentation, physical examination, and imaging features are all important for a proper evaluation and optimal management.
- Position of play is an important consideration in the management of upper extremity injuries in soccer. Outfield players may be able to return to play before a complete resolution of their injury, with protective accessories.
- Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
Medical Complications and Outcomes After Total Shoulder Arthroplasty: A Nationwide Analysis
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
TAKE HOME POINTS
- Medical complications are common (6.7%) after total shoulder arthroplasty.
- Age and preoperative medical comorbidities increased the risk of a postoperative complication.
- The most frequent medical complications are respiratory, renal, and cardiac.
- Length of stay was effected most by shock, infections, and vascular complications.
- Mortality was associated with major complications such as, shock, central nervous system, cardiac, vascular, and respiratory complications.
Reverse Total Shoulder Arthroplasty: Indications and Techniques Across the World
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
TAKE-HOME POINTS
- RTSA is an effective treatment for rotator cuff tear arthropathy (the most common reason patients undergo RTSA).
- While there has been a plethora of literature surrounding outcomes of RTSA over the past several years, the methodological quality of this literature has been limited.
- Similarly, this study found the number of publications surrounding RTSA is increasing each year while the average methodological quality of these studies is decreasing.
- Females undergo RTSA more commonly than males, and the average age of patients undergoing RTSA is 71 years.
- Interestingly, patients’ postoperative external rotation was higher in studies out of North America compared to other continents. Further research into this area is needed to understand more about this finding.
Arthroscopically-Guided, Cannulated, Headless Compression Screw Fixation of the Symptomatic Os Acromiale
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
TAKE-HOME POINTS
- Os acromiale is a failure of acromial ossification centers to fuse, and occurs in 8% of the population.
- Symptomatic os acromiale can be treated with repair, or sometimes excision or acromioplasty.
- Repair preserves the anterior deltoid origin and can result in less pain than excision of the fragment.
- Repair of larger fragments can be completed with cannulated screws to reliably achieve union.
- The arthroscope-assisted repair technique described in this article preserves vascularity and can reduce the risk of hardware-related complaints.
Subcutaneous Ulnar Nerve Transposition Using Osborne’s Ligament as a Ligamentodermal or Ligamentofascial Sling
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
TAKE-HOME POINTS
- Optimal management of cubital tunnel syndrome is controversial.
- There are many different techniques for ulnar nerve transposition, each with their own set of pitfalls.
- Goal of any surgery for ulnar nerve compression is to eliminate all sites of compression and create a tension-free nerve that glides freely.
- Osborne’s ligament is a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel.
- Osborne’s ligament can be used in ulnar nerve transposition to create a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve.
Case Series Evaluating the Operative and Nonoperative Treatment of Scapular Fractures
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.
Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.
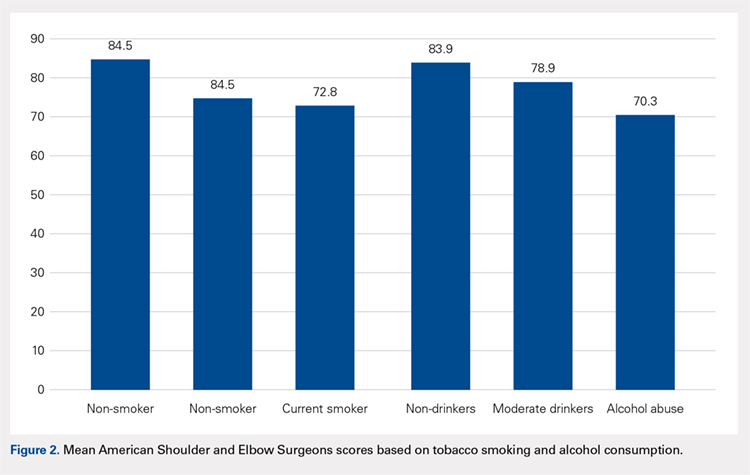
Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
TAKE-HOME POINTS
- The majority of patients with scapula fractures are multiply-injured.
- Despite being multiply-injured, most heal with minimal functional shoulder impairment.
- While concomitant injuries do not appear to affect shoulder function scores, tobacco use and alcohol abuse are associated with worse outcomes after scapula fractures.
- Most scapula fractures can be treated successfully without surgery.
- Although patients had higher average function scores after open reduction and internal fixation, further research should be done to define indications for fixation.