User login
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
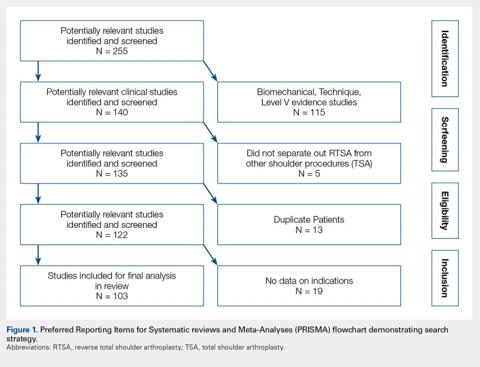
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
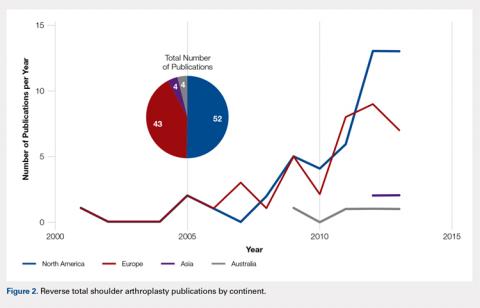
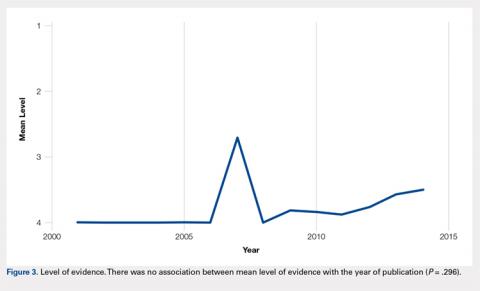
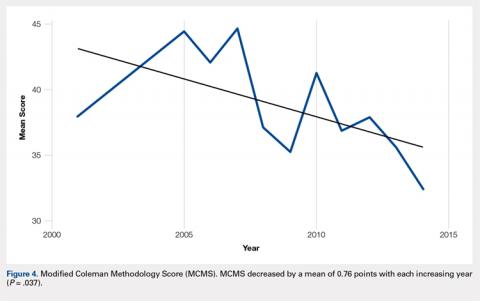
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
TAKE-HOME POINTS
- RTSA is an effective treatment for rotator cuff tear arthropathy (the most common reason patients undergo RTSA).
- While there has been a plethora of literature surrounding outcomes of RTSA over the past several years, the methodological quality of this literature has been limited.
- Similarly, this study found the number of publications surrounding RTSA is increasing each year while the average methodological quality of these studies is decreasing.
- Females undergo RTSA more commonly than males, and the average age of patients undergoing RTSA is 71 years.
- Interestingly, patients’ postoperative external rotation was higher in studies out of North America compared to other continents. Further research into this area is needed to understand more about this finding.