User login
Reverse Total Shoulder Arthroplasty: Indications and Techniques Across the World
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
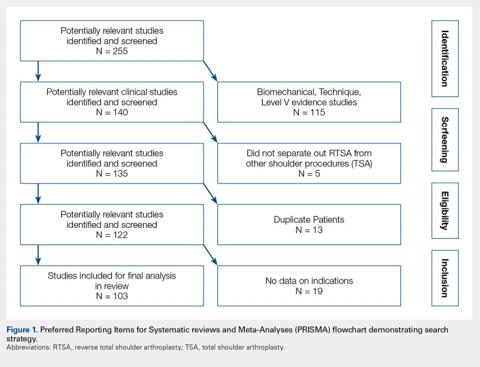
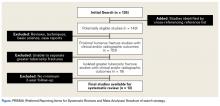
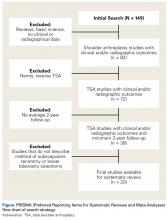
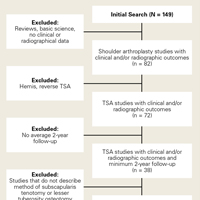
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
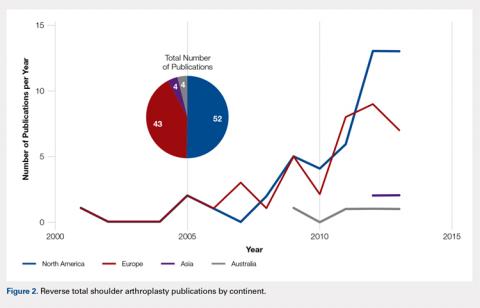
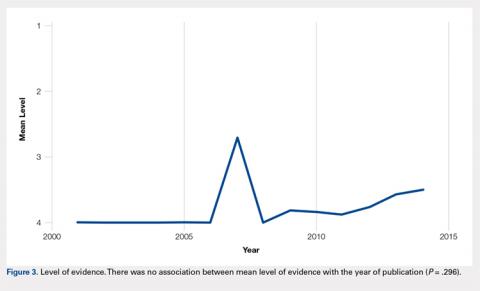
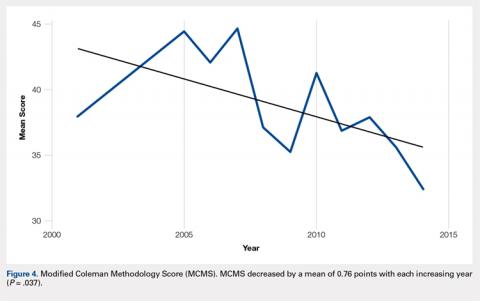
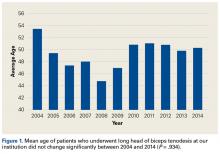
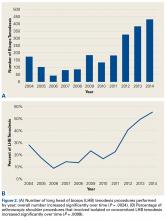
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
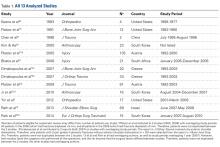
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
TAKE-HOME POINTS
- RTSA is an effective treatment for rotator cuff tear arthropathy (the most common reason patients undergo RTSA).
- While there has been a plethora of literature surrounding outcomes of RTSA over the past several years, the methodological quality of this literature has been limited.
- Similarly, this study found the number of publications surrounding RTSA is increasing each year while the average methodological quality of these studies is decreasing.
- Females undergo RTSA more commonly than males, and the average age of patients undergoing RTSA is 71 years.
- Interestingly, patients’ postoperative external rotation was higher in studies out of North America compared to other continents. Further research into this area is needed to understand more about this finding.
Reasons for Readmission Following Primary Total Shoulder Arthroplasty
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
TAKE-HOME POINTS
- Shoulder arthroplasty is an increasingly commonly performed procedure for shoulder arthritis and other conditions.
- Unplanned readmission in the 30 days after shoulder arthroplasty occurred in about 1 of 40 cases.
- Increasing age was associated with readmission, particularly age >80 years.
- Other risk factors for readmission were male sex, anemia, and dependent functional status.
- The most common reasons for readmission were pneumonia, dislocation, pulmonary embolism, and surgical site infection.
Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Population-Based Study Examining Utilization, Adverse Events, Length of Stay, and Cost
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).
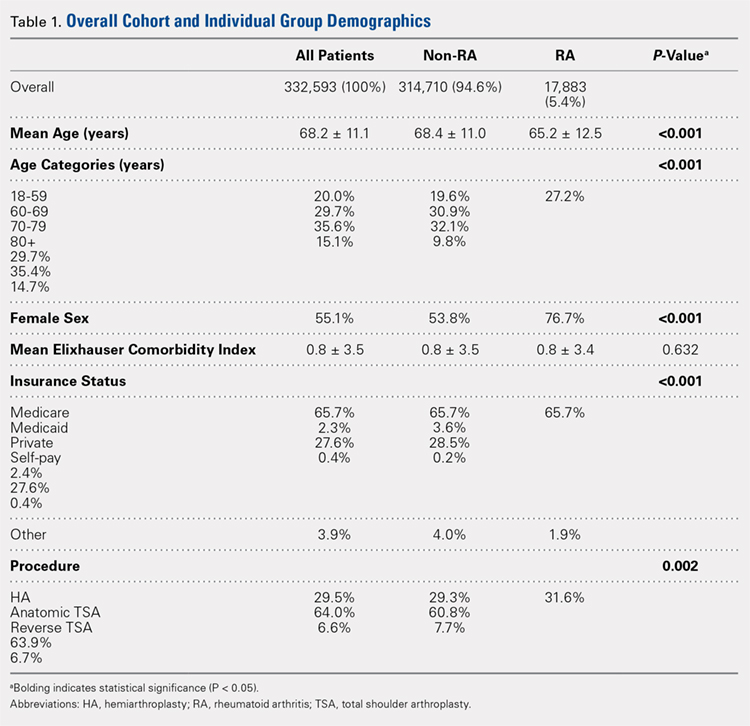
Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.
Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
TAKE-HOME POINTS
- There was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly TSA.
- There was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease.
- There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients.
- Non-RA patients had a significantly shorter length of stay.
- The utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Impact of Sagittal Rotation on Axial Glenoid Width Measurement in the Setting of Glenoid Bone Loss
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
TAKE-HOME POINTS
- Standard 2-D CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and GBL.
- Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity.
- AP glenoid width was consistently underestimated in uncorrected axial cut 1, the most caudal cut.
- AP glenoid width was consistently overestimated in uncorrected axial cuts 3 to 5, the more cephalad cuts.
- CORR 2-D CT scans or a 3-D reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
Management of Isolated Greater Tuberosity Fractures: A Systematic Review
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Biceps Tenodesis: An Evolution of Treatment
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
Subscapularis Tenotomy Versus Lesser Tuberosity Osteotomy for Total Shoulder Arthroplasty: A Systematic Review
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Management of Proximal Biceps Pathology in Overhead Athletes: What Is the Role of Biceps Tenodesis?
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.