User login
Visionary Surgery Saved Pitcher’s Arm. Now Even Children Get It
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
High school athletes sustaining worse injuries
High school students are injuring themselves more severely even as overall injury rates have declined, according to a new study presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
The study compared injuries from a 4-year period ending in 2019 to data from 2005 and 2006. The overall rate of injuries dropped 9%, from 2.51 injuries per 1,000 athletic games or practices to 2.29 per 1,000; injuries requiring less than 1 week of recovery time fell by 13%. But, the number of head and neck injuries increased by 10%, injuries requiring surgery increased by 1%, and injuries leading to medical disqualification jumped by 11%.
“It’s wonderful that the injury rate is declining,” said Jordan Neoma Pizzarro, a medical student at George Washington University, Washington, who led the study. “But the data does suggest that the injuries that are happening are worse.”
The increases may also reflect increased education and awareness of how to detect concussions and other injuries that need medical attention, said Micah Lissy, MD, MS, an orthopedic surgeon specializing in sports medicine at Michigan State University, East Lansing. Dr. Lissy cautioned against physicians and others taking the data at face value.
“We need to be implementing preventive measures wherever possible, but I think we can also consider that there may be some confounding factors in the data,” Dr. Lissy told this news organization.
Ms. Pizzarro and her team analyzed data collected from athletic trainers at 100 high schools across the country for the ongoing National Health School Sports-Related Injury Surveillance Study.
Athletes participating in sports such as football, soccer, basketball, volleyball, and softball were included in the analysis. Trainers report the number of injuries for every competition and practice, also known as “athletic exposures.”
Boys’ football carried the highest injury rate, with 3.96 injuries per 1,000 AEs, amounting to 44% of all injuries reported. Girls’ soccer and boys’ wrestling followed, with injury rates of 2.65 and 1.56, respectively.
Sprains and strains accounted for 37% of injuries, followed by concussions (21.6%). The head and/or face was the most injured body site, followed by the ankles and/or knees. Most injuries took place during competitions rather than in practices (relative risk, 3.39; 95% confidence interval, 3.28-3.49; P < .05).
Ms. Pizzarro said that an overall increase in intensity, physical contact, and collisions may account for the spike in more severe injuries.
“Kids are encouraged to specialize in one sport early on and stick with it year-round,” she said. “They’re probably becoming more agile and better athletes, but they’re probably also getting more competitive.”
Dr. Lissy, who has worked with high school athletes as a surgeon, physical therapist, athletic trainer, and coach, said that some of the increases in severity of injuries may reflect trends in sports over the past two decades: Student athletes have become stronger and faster and have put on more muscle mass.
“When you have something that’s much larger, moving much faster and with more force, you’re going to have more force when you bump into things,” he said. “This can lead to more significant injuries.”
The study was independently supported. Study authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High school students are injuring themselves more severely even as overall injury rates have declined, according to a new study presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
The study compared injuries from a 4-year period ending in 2019 to data from 2005 and 2006. The overall rate of injuries dropped 9%, from 2.51 injuries per 1,000 athletic games or practices to 2.29 per 1,000; injuries requiring less than 1 week of recovery time fell by 13%. But, the number of head and neck injuries increased by 10%, injuries requiring surgery increased by 1%, and injuries leading to medical disqualification jumped by 11%.
“It’s wonderful that the injury rate is declining,” said Jordan Neoma Pizzarro, a medical student at George Washington University, Washington, who led the study. “But the data does suggest that the injuries that are happening are worse.”
The increases may also reflect increased education and awareness of how to detect concussions and other injuries that need medical attention, said Micah Lissy, MD, MS, an orthopedic surgeon specializing in sports medicine at Michigan State University, East Lansing. Dr. Lissy cautioned against physicians and others taking the data at face value.
“We need to be implementing preventive measures wherever possible, but I think we can also consider that there may be some confounding factors in the data,” Dr. Lissy told this news organization.
Ms. Pizzarro and her team analyzed data collected from athletic trainers at 100 high schools across the country for the ongoing National Health School Sports-Related Injury Surveillance Study.
Athletes participating in sports such as football, soccer, basketball, volleyball, and softball were included in the analysis. Trainers report the number of injuries for every competition and practice, also known as “athletic exposures.”
Boys’ football carried the highest injury rate, with 3.96 injuries per 1,000 AEs, amounting to 44% of all injuries reported. Girls’ soccer and boys’ wrestling followed, with injury rates of 2.65 and 1.56, respectively.
Sprains and strains accounted for 37% of injuries, followed by concussions (21.6%). The head and/or face was the most injured body site, followed by the ankles and/or knees. Most injuries took place during competitions rather than in practices (relative risk, 3.39; 95% confidence interval, 3.28-3.49; P < .05).
Ms. Pizzarro said that an overall increase in intensity, physical contact, and collisions may account for the spike in more severe injuries.
“Kids are encouraged to specialize in one sport early on and stick with it year-round,” she said. “They’re probably becoming more agile and better athletes, but they’re probably also getting more competitive.”
Dr. Lissy, who has worked with high school athletes as a surgeon, physical therapist, athletic trainer, and coach, said that some of the increases in severity of injuries may reflect trends in sports over the past two decades: Student athletes have become stronger and faster and have put on more muscle mass.
“When you have something that’s much larger, moving much faster and with more force, you’re going to have more force when you bump into things,” he said. “This can lead to more significant injuries.”
The study was independently supported. Study authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High school students are injuring themselves more severely even as overall injury rates have declined, according to a new study presented at the annual meeting of the American Academy of Orthopaedic Surgeons.
The study compared injuries from a 4-year period ending in 2019 to data from 2005 and 2006. The overall rate of injuries dropped 9%, from 2.51 injuries per 1,000 athletic games or practices to 2.29 per 1,000; injuries requiring less than 1 week of recovery time fell by 13%. But, the number of head and neck injuries increased by 10%, injuries requiring surgery increased by 1%, and injuries leading to medical disqualification jumped by 11%.
“It’s wonderful that the injury rate is declining,” said Jordan Neoma Pizzarro, a medical student at George Washington University, Washington, who led the study. “But the data does suggest that the injuries that are happening are worse.”
The increases may also reflect increased education and awareness of how to detect concussions and other injuries that need medical attention, said Micah Lissy, MD, MS, an orthopedic surgeon specializing in sports medicine at Michigan State University, East Lansing. Dr. Lissy cautioned against physicians and others taking the data at face value.
“We need to be implementing preventive measures wherever possible, but I think we can also consider that there may be some confounding factors in the data,” Dr. Lissy told this news organization.
Ms. Pizzarro and her team analyzed data collected from athletic trainers at 100 high schools across the country for the ongoing National Health School Sports-Related Injury Surveillance Study.
Athletes participating in sports such as football, soccer, basketball, volleyball, and softball were included in the analysis. Trainers report the number of injuries for every competition and practice, also known as “athletic exposures.”
Boys’ football carried the highest injury rate, with 3.96 injuries per 1,000 AEs, amounting to 44% of all injuries reported. Girls’ soccer and boys’ wrestling followed, with injury rates of 2.65 and 1.56, respectively.
Sprains and strains accounted for 37% of injuries, followed by concussions (21.6%). The head and/or face was the most injured body site, followed by the ankles and/or knees. Most injuries took place during competitions rather than in practices (relative risk, 3.39; 95% confidence interval, 3.28-3.49; P < .05).
Ms. Pizzarro said that an overall increase in intensity, physical contact, and collisions may account for the spike in more severe injuries.
“Kids are encouraged to specialize in one sport early on and stick with it year-round,” she said. “They’re probably becoming more agile and better athletes, but they’re probably also getting more competitive.”
Dr. Lissy, who has worked with high school athletes as a surgeon, physical therapist, athletic trainer, and coach, said that some of the increases in severity of injuries may reflect trends in sports over the past two decades: Student athletes have become stronger and faster and have put on more muscle mass.
“When you have something that’s much larger, moving much faster and with more force, you’re going to have more force when you bump into things,” he said. “This can lead to more significant injuries.”
The study was independently supported. Study authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Evaluation of the American Academy of Orthopaedic Surgeons Appropriate Use Criteria for the Nonarthroplasty Treatment of Knee Osteoarthritis in Veterans
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
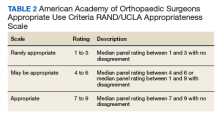
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
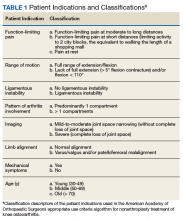
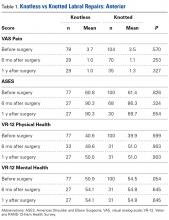
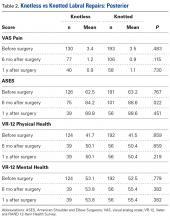
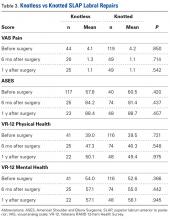
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
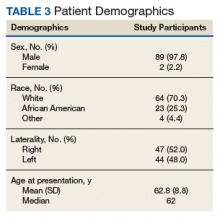
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Preoperative Corticosteroid Use for Medical Conditions is Associated with Increased Postoperative Infectious Complications and Readmissions After Total Hip Arthroplasty: A Propensity-Matched Study
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.
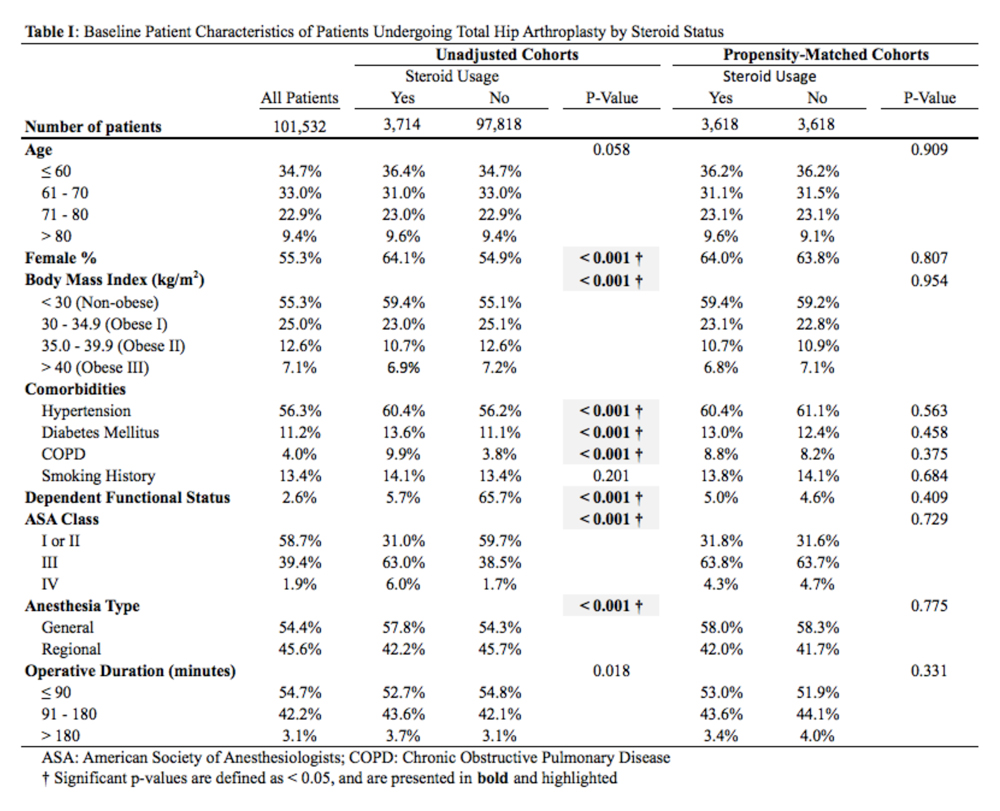
When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).
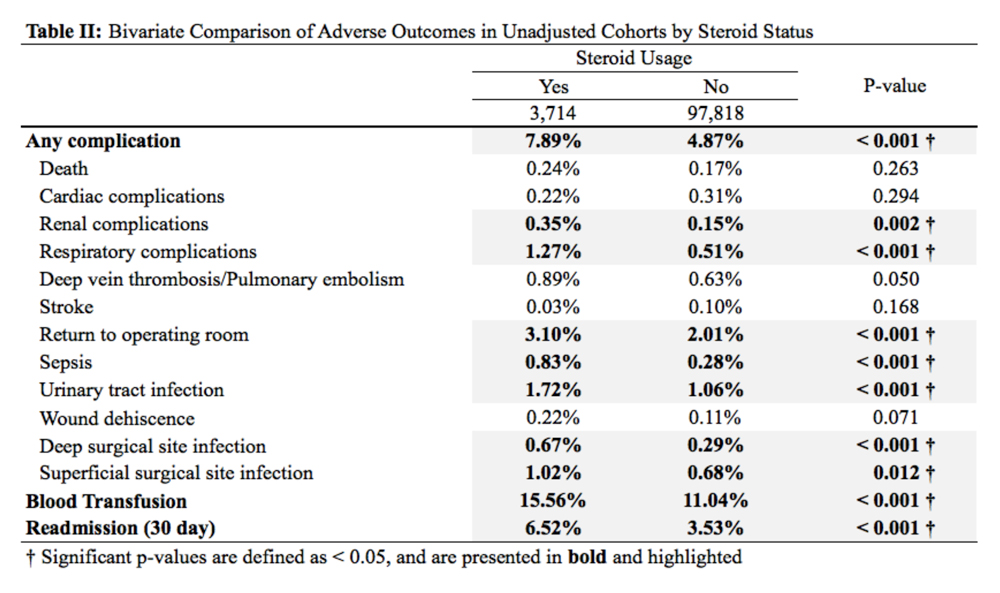
Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).
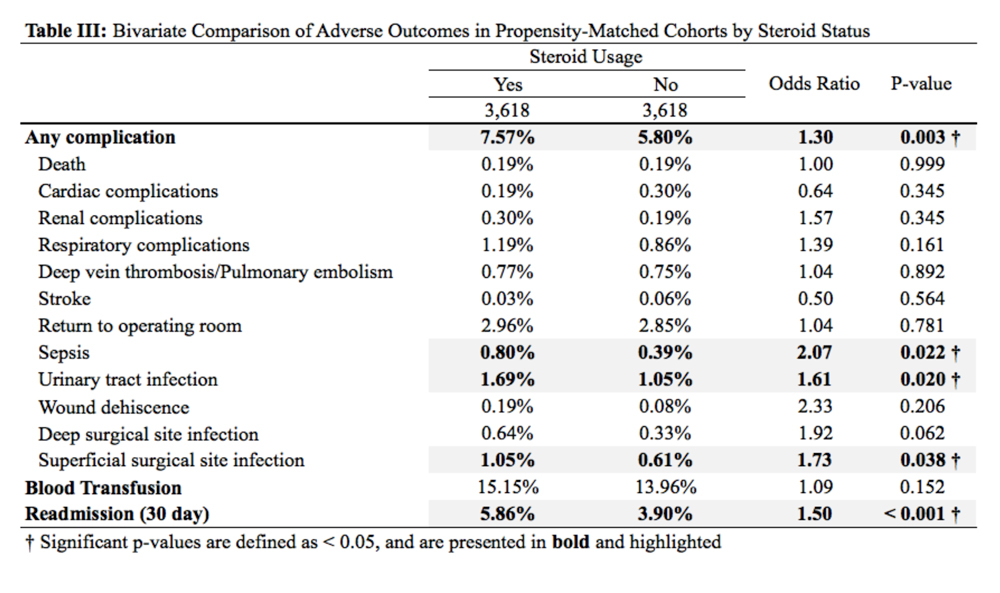
REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).
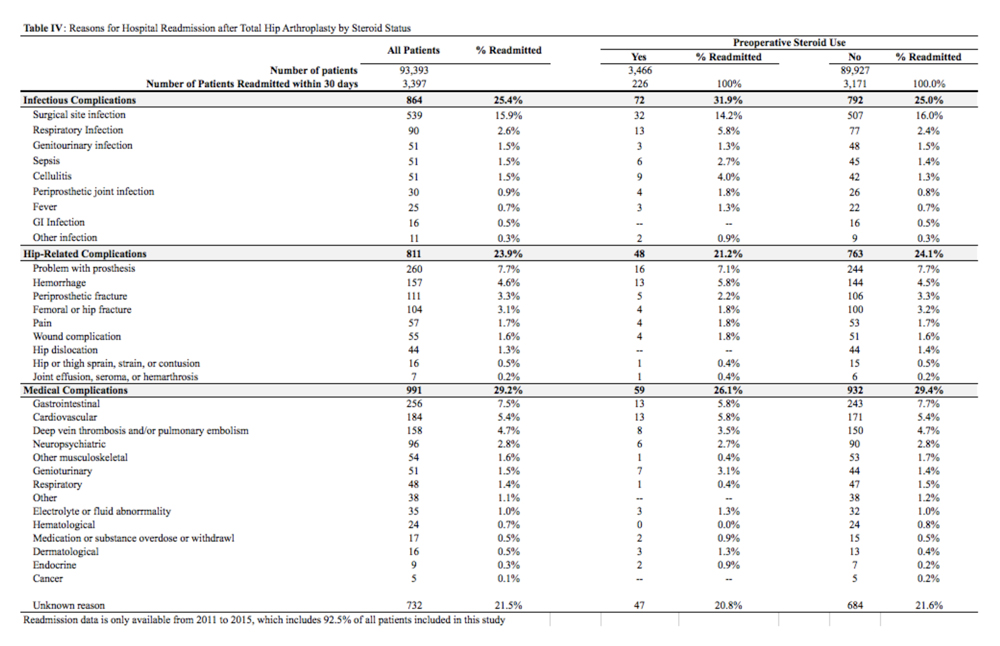
The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.

When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).

Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).

REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).

The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.

When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).

Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).

REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).

The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
TAKE-HOME POINTS
- The rate of preoperative corticosteroid usage is low (3.7%).
- Patients using preoperative corticosteroids had increased rates of total 30-day complications.
- Adverse outcomes that are increased include infectious complications (eg, sepsis, urinary tract infection, surgical site infection).
- Hospital readmissions are also increased in patients taking preoperative corticosteroids, with the most common reason being infection.
- Increased postoperative counseling and surveillance may be warranted in this patient population.
Geniculate Artery Injury During Primary Total Knee Arthroplasty
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
TAKE-HOME POINTS
- During total knee arthroscopy (TKA), 38% of patients will have an injury of a geniculate artery.
- The lateral inferior geniculate artery is most commonly injured, with a rate of injury of 31%.
- The middle geniculate artery is injured 15% of the time.
- The most common time of geniculate artery injury is during bone cutting or removal of the meniscus.
- There is no difference in rate of geniculate artery injury identification with or without the use of a tourniquet.
Participation in Work and Sport Following Reverse and Total Shoulder Arthroplasty
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
TAKE-HOME POINTS
- Both anatomic (TSA) and reverse shoulder arthroplasty (RSA) allow for the participation in work and sports.
- TSA patients report easier overall ability to participate in sports, specifically golf and swimming.
- For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
- TSA patients report easier overall ability to return to work-related activities, specifically housework and gardening.
- TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients.
Patient-Reported Outcomes of Knotted and Knotless Glenohumeral Labral Repairs Are Equivalent
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
In-Office Diagnostic Needle Arthroscopy: Understanding the Potential Value for the US Healthcare System
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Practice Makes Perfect?
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.