User login
5 Points on Meniscal Allograft Transplantation
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
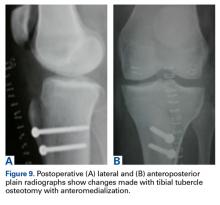
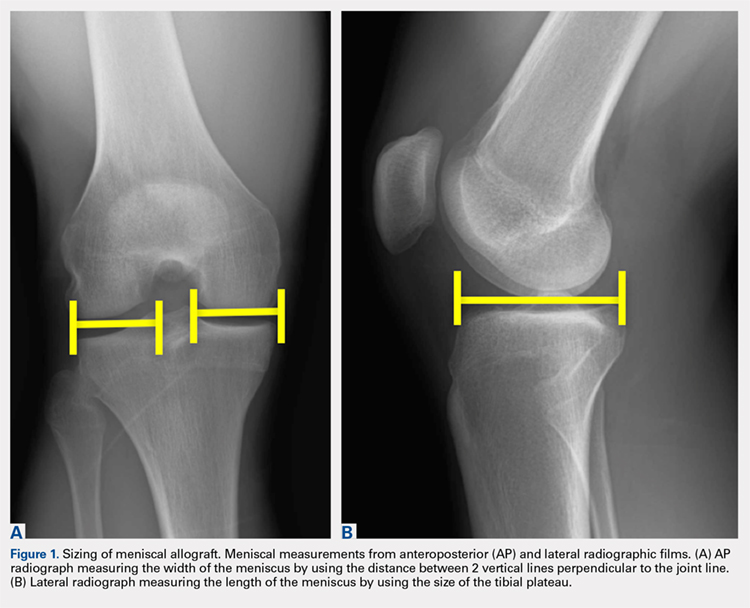
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.
Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
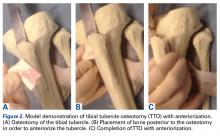
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.
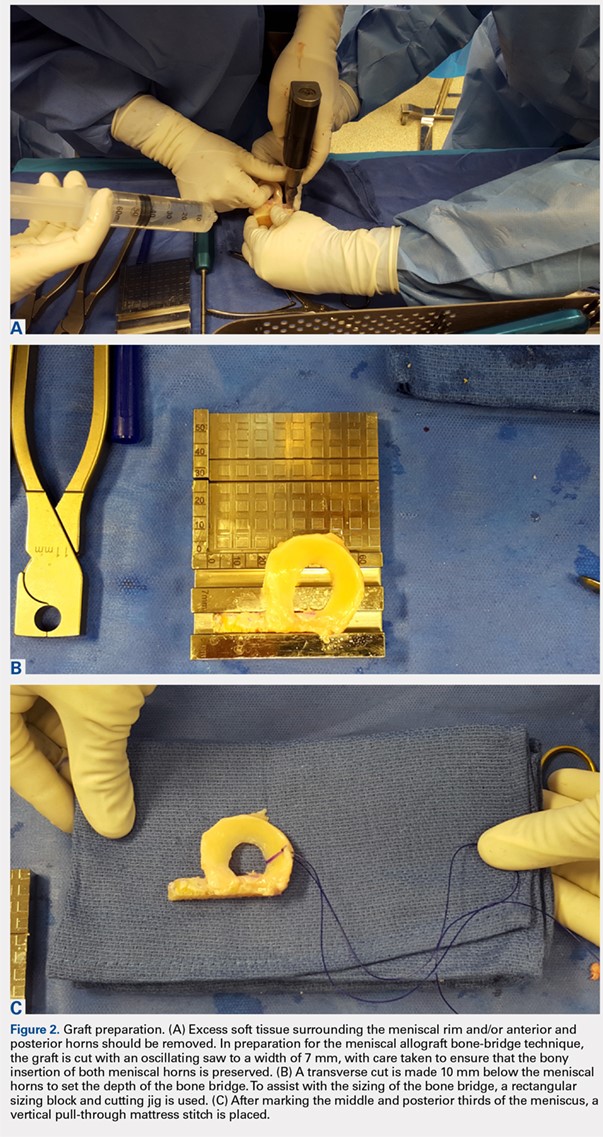
Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
TAKE-HOME POINTS
- Patient selection is critical for obtaining long-term functional outcome improvements and reduced pain, with the ideal MAT candidate being a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency.
- Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure.
- Accurate graft width and length measurements are vital, and the most common technique used today includes measuring the meniscus on anteroposterior and lateral radiographic images.
- When preparing the graft for the bone-bridge technique, the bone is fashioned to create a bone bridge 10 mm in depth by approximately 7 mm in width, incorporating the anterior and posterior horns of the meniscus.
- Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Reasons for Readmission Following Primary Total Shoulder Arthroplasty
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
TAKE-HOME POINTS
- Shoulder arthroplasty is an increasingly commonly performed procedure for shoulder arthritis and other conditions.
- Unplanned readmission in the 30 days after shoulder arthroplasty occurred in about 1 of 40 cases.
- Increasing age was associated with readmission, particularly age >80 years.
- Other risk factors for readmission were male sex, anemia, and dependent functional status.
- The most common reasons for readmission were pneumonia, dislocation, pulmonary embolism, and surgical site infection.
Reoperation Rates After Cartilage Restoration Procedures in the Knee: Analysis of a Large US Commercial Database
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).
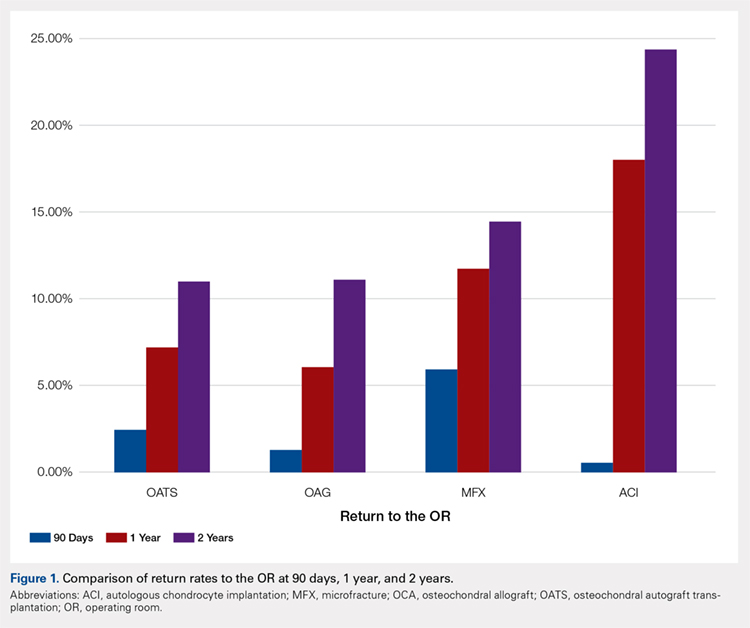
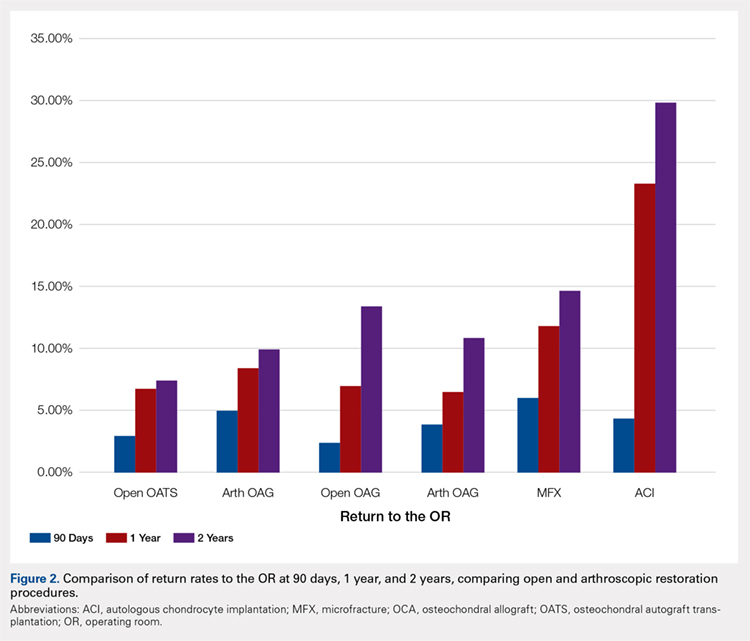
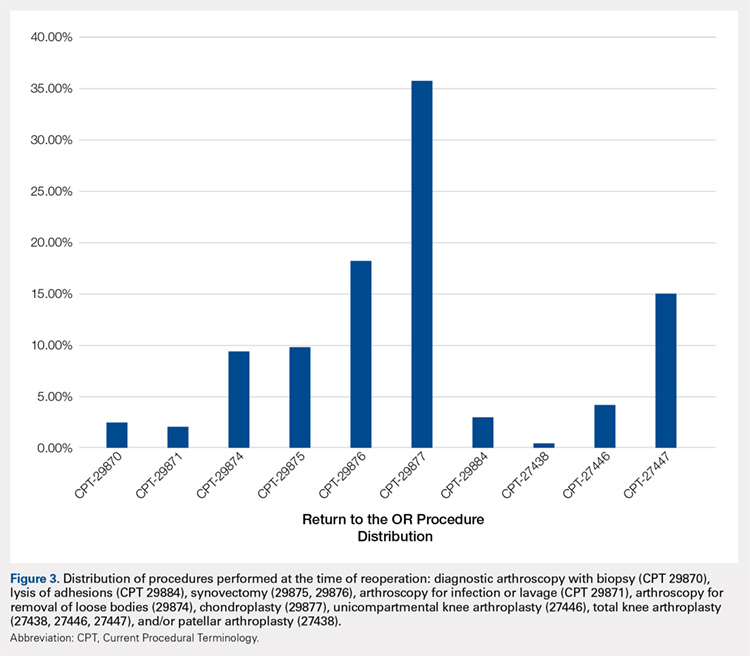
Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
TAKE-HOME POINTS
- With a large US commercial insurance database analyzing techniques for cartilage restoration, reparative procedures were favored for chondral injuries compared to restorative approaches.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 90-day reoperation rate is 6%.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 2-year reoperation rate is 15%.
- Patients undergoing autologous chondrocyte implantation are more likely to experience reoperation at 90 days, 1 year, and 2 years compared to other cartilage restoration techniques including microfracture, osteochondral autograft transfer, and osteochondral allograft transplantation.
- Patients undergoing microfracture are more likely to experience an ultimate conversion to arthroplasty compared to other cartilage restoration techniques including autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation.
A Systematic Review of 21 Tibial Tubercle Osteotomy Studies and More Than 1000 Knees: Indications, Clinical Outcomes, Complications, and Reoperations
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.