User login
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
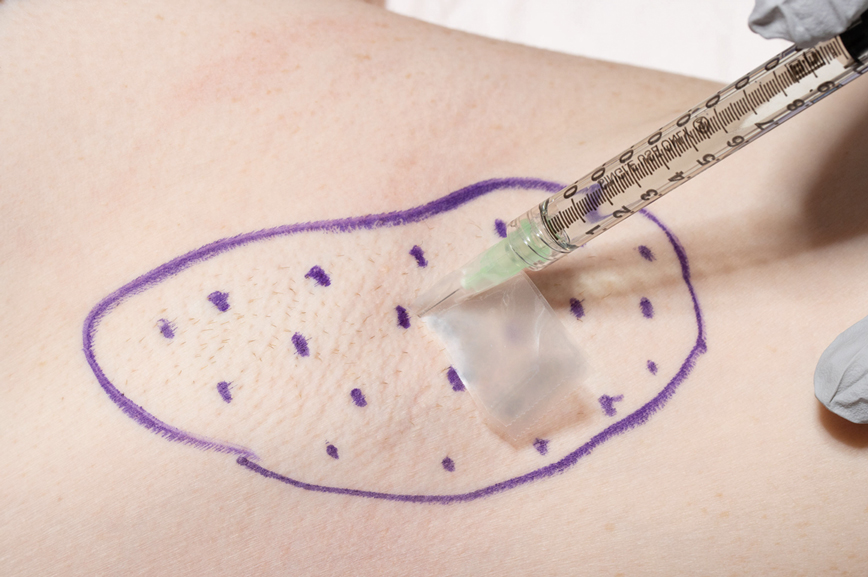
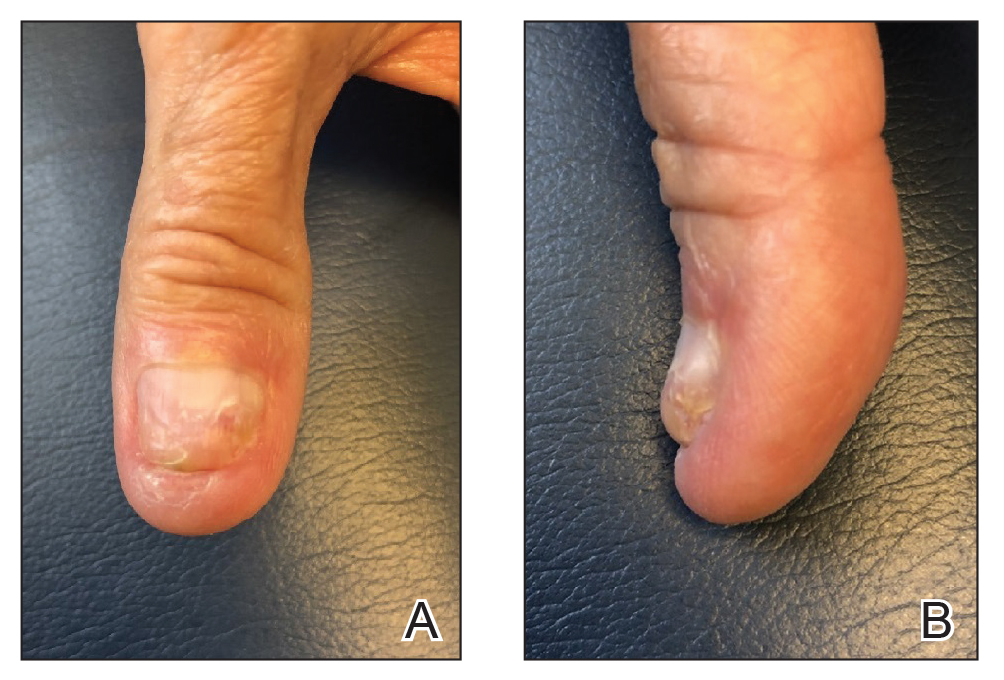
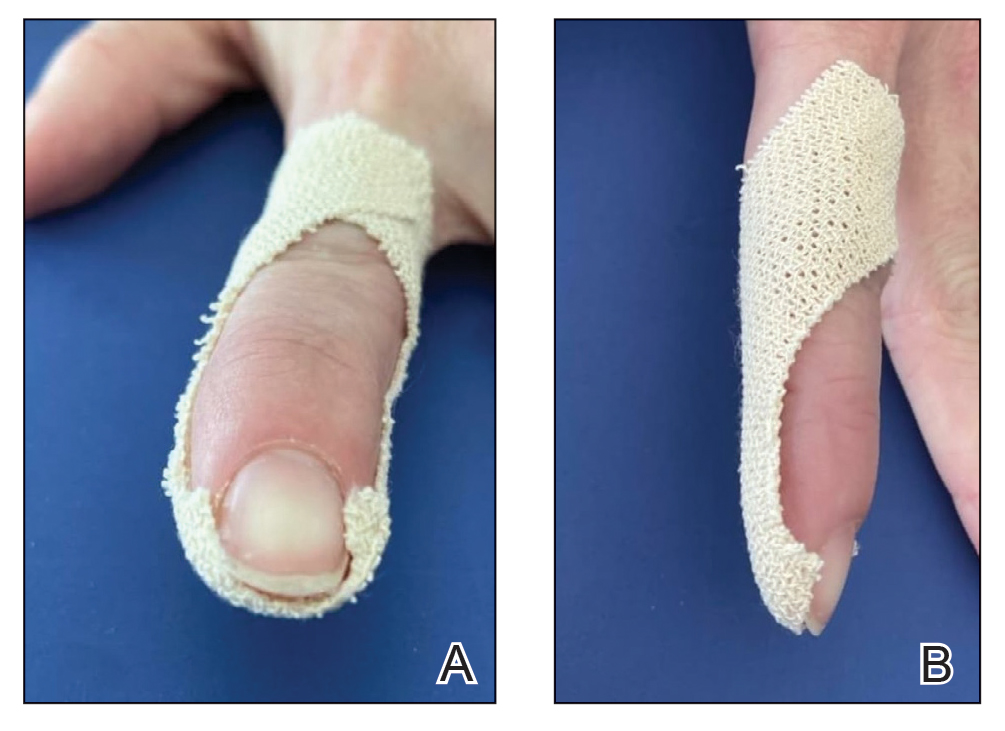
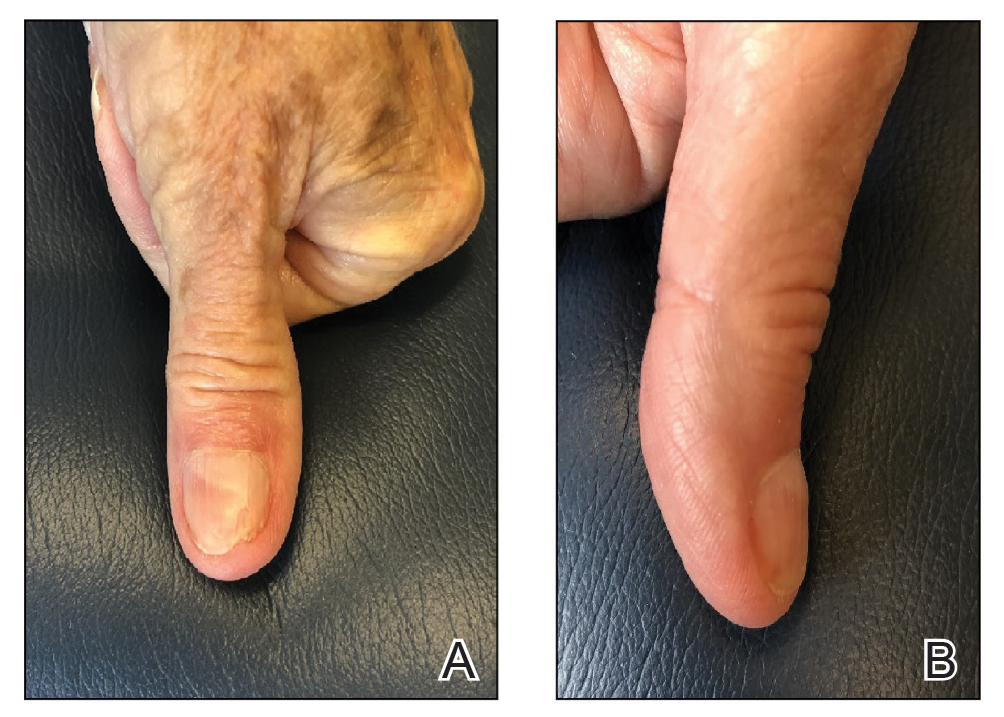
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Adhesive Tape to Guide Injection Depth of Botulinum Toxin for Axillary Hyperhidrosis
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
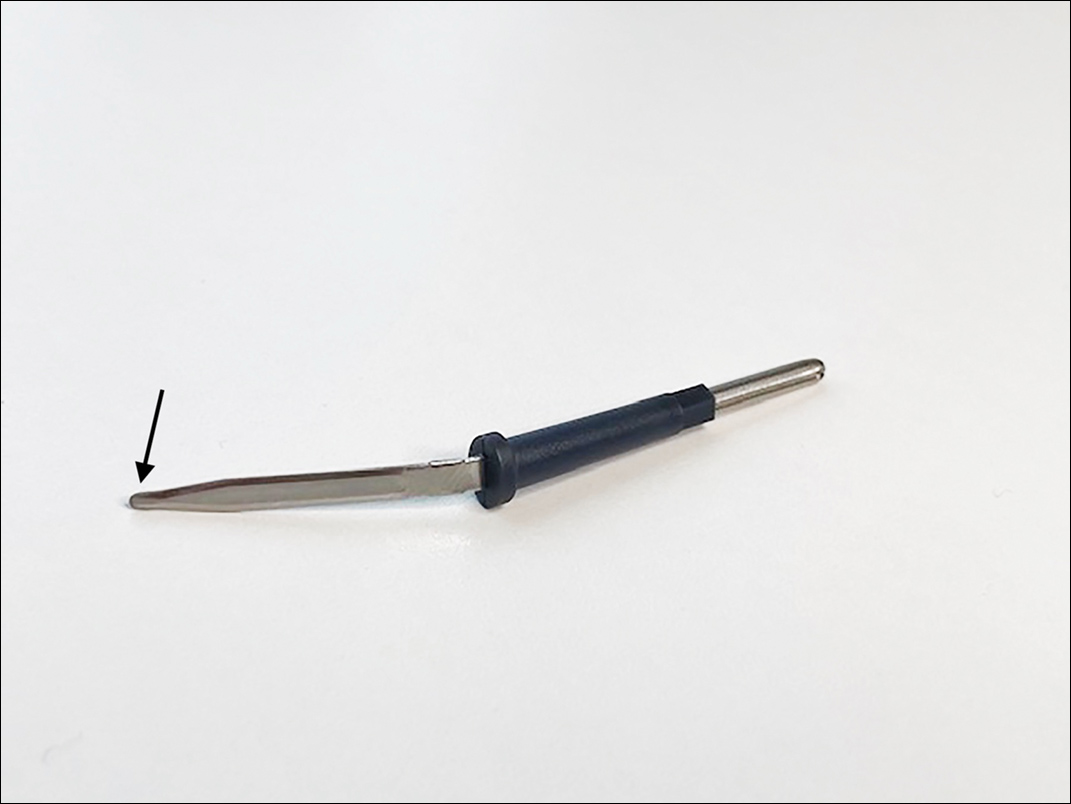
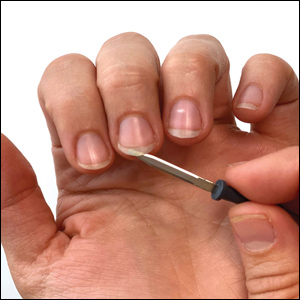
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
Reflectance Confocal Microscopy Findings in a Small-Diameter Invasive Melanoma
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
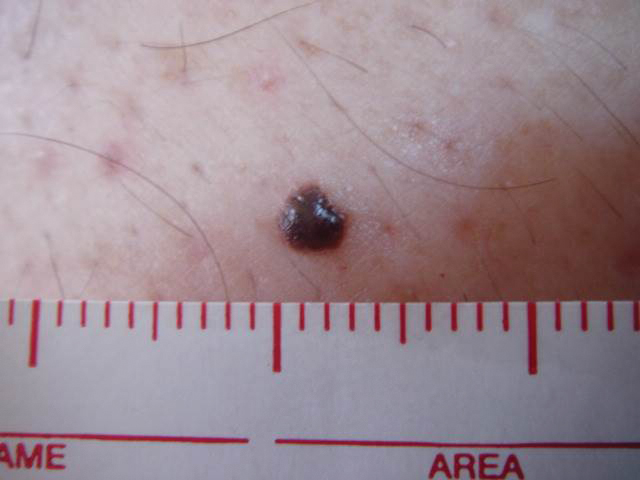
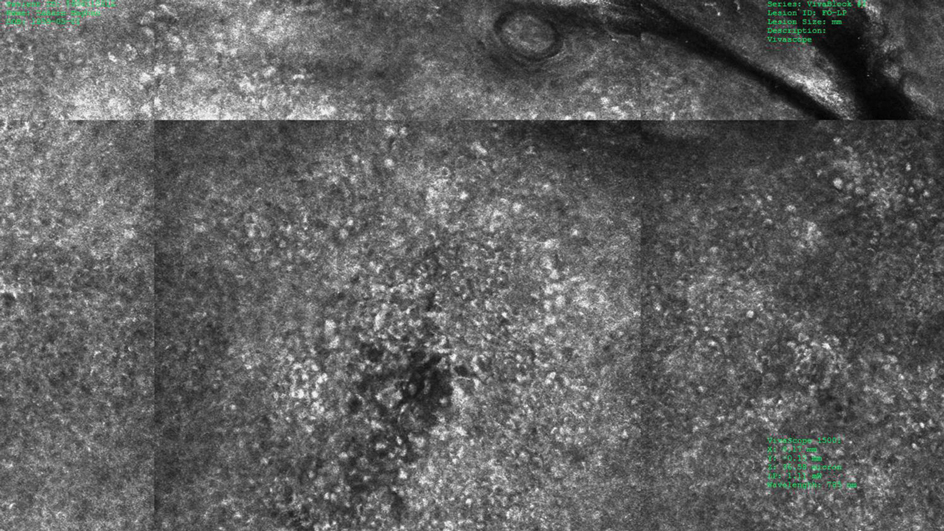
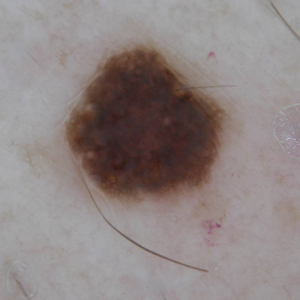
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
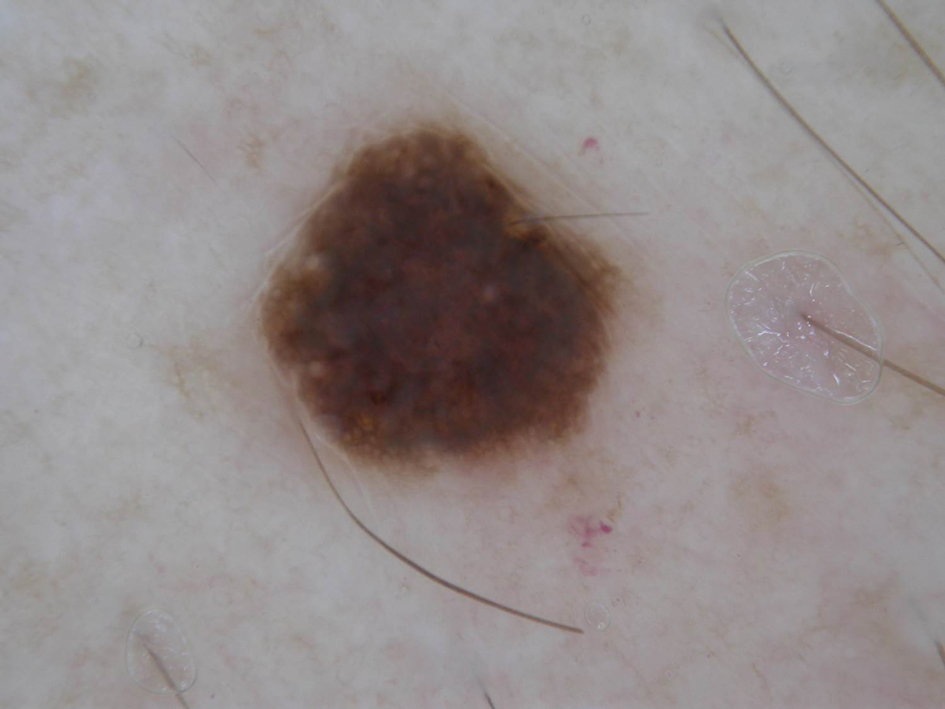
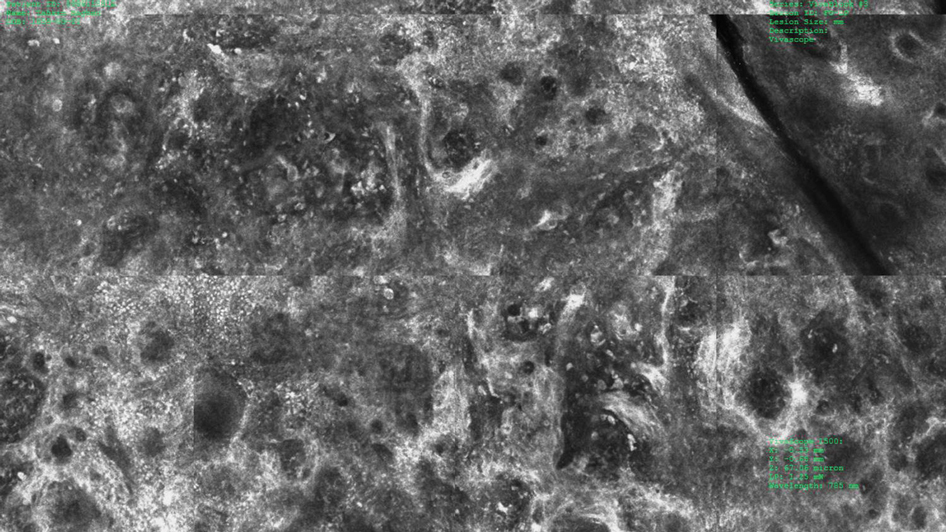
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
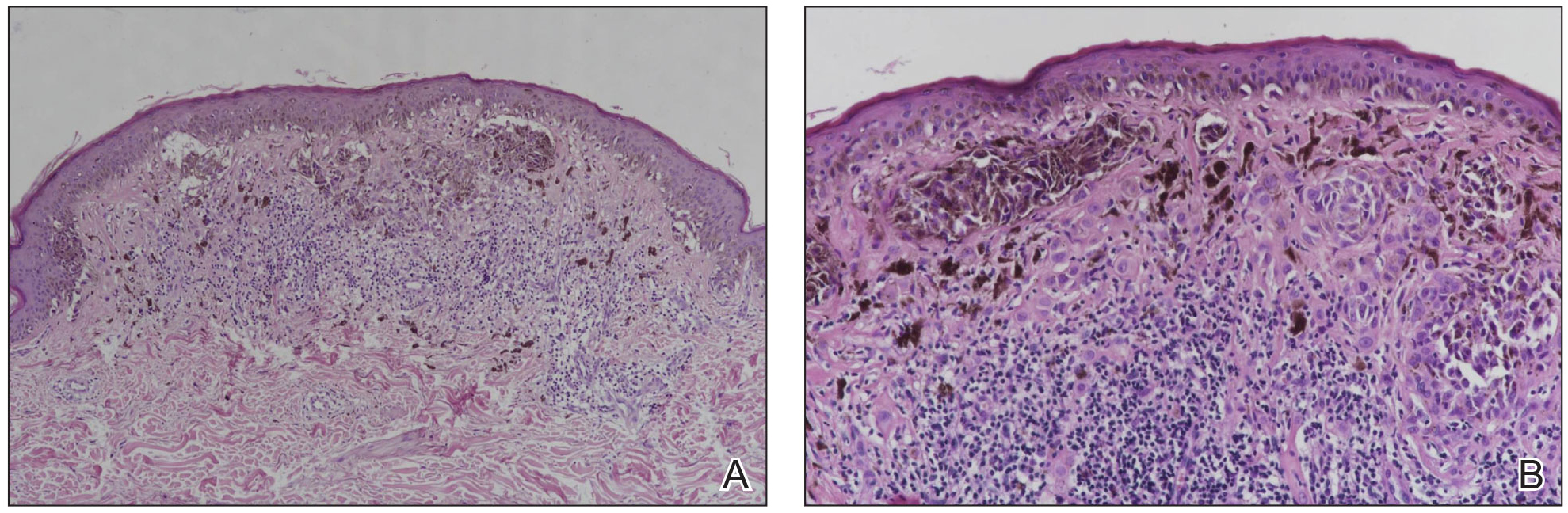
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.
The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Practice Points
- Melanomas with a long-axis diameter smaller than 6 mm are considered small melanomas, and those with diameters of 3 mm and smaller are considered micromelanomas; both are difficult to detect.
- Digital dermoscopic monitoring and reflectance confocal microscopy are important tools in detecting small melanomas.
Surgical Planning for Mohs Defect Reconstruction in the Digital Age
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Wet Your Whistles: Alcohol-Induced Flushing With Use of Topical Calcineurin Inhibitors
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
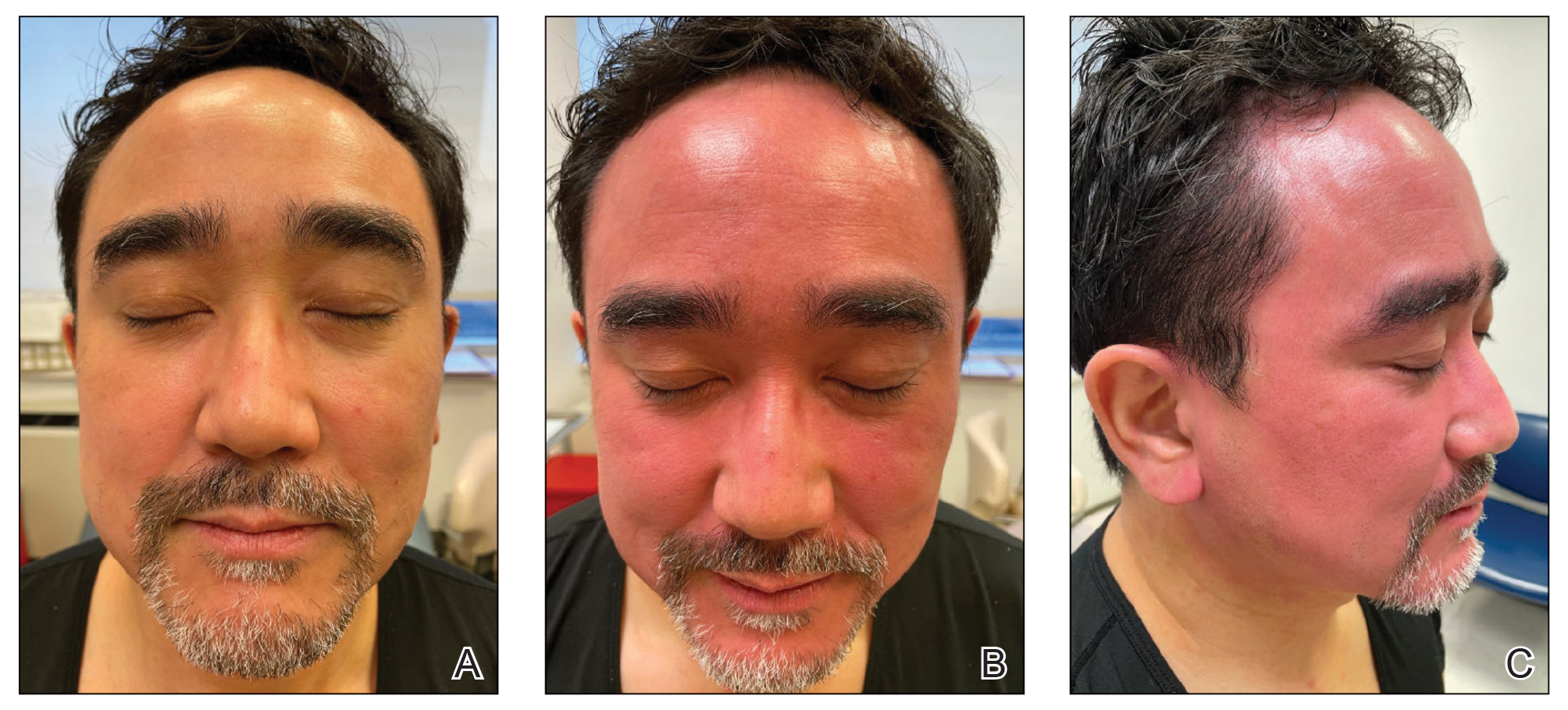
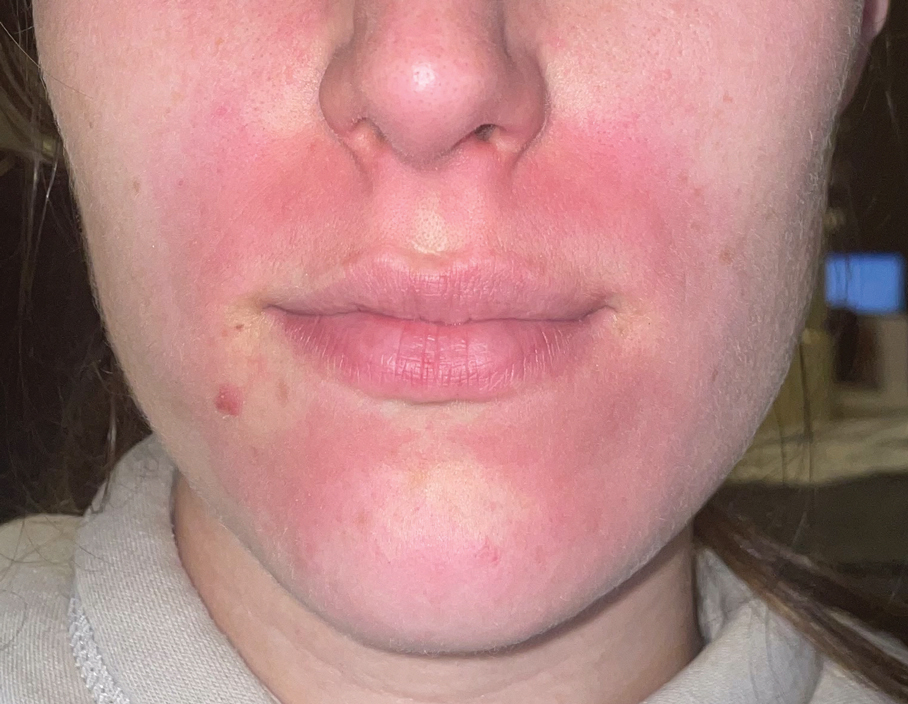
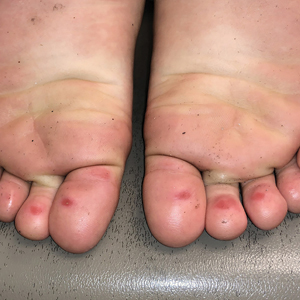
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.
Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.
Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.
Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.
Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.
Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.
Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
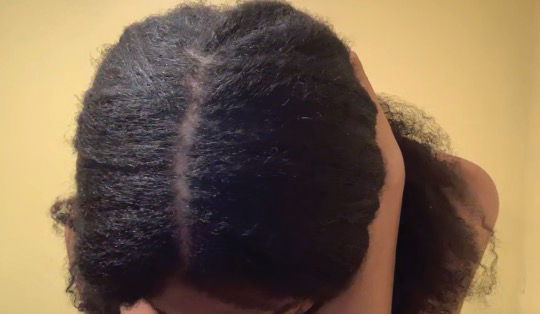
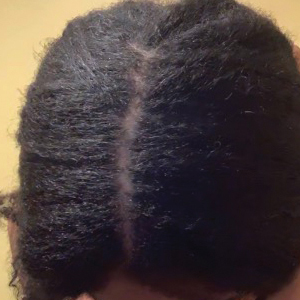
Hairstyling Practices to Prevent Hair Damage and Alopecia in Women of African Descent
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Not COVID Toes: Pool Palms and Feet in Pediatric Patients
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
Mineral Oil Scabies Preparation From Under the Fingernail
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.
At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.
The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.
Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.
At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.
The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.
Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.
At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.
The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.
Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
A Modified Anchor Taping Technique for Distal Onychocryptosis
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.