User login
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2
Technique
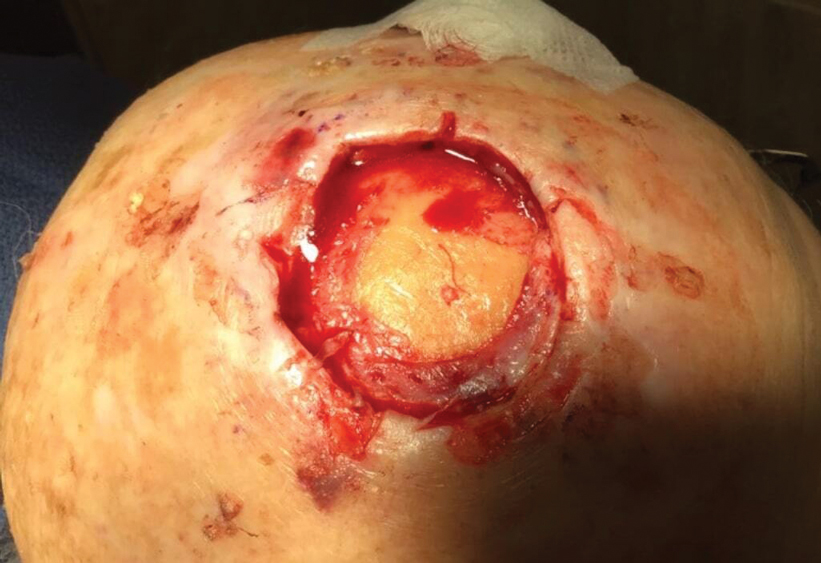
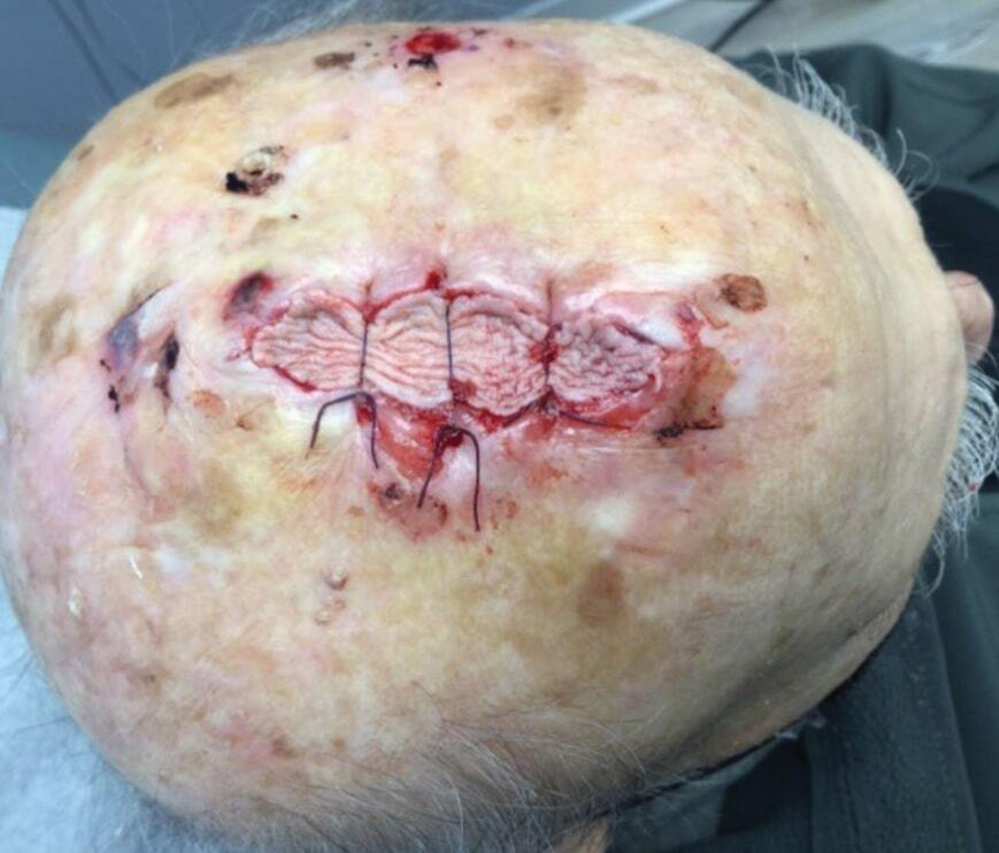
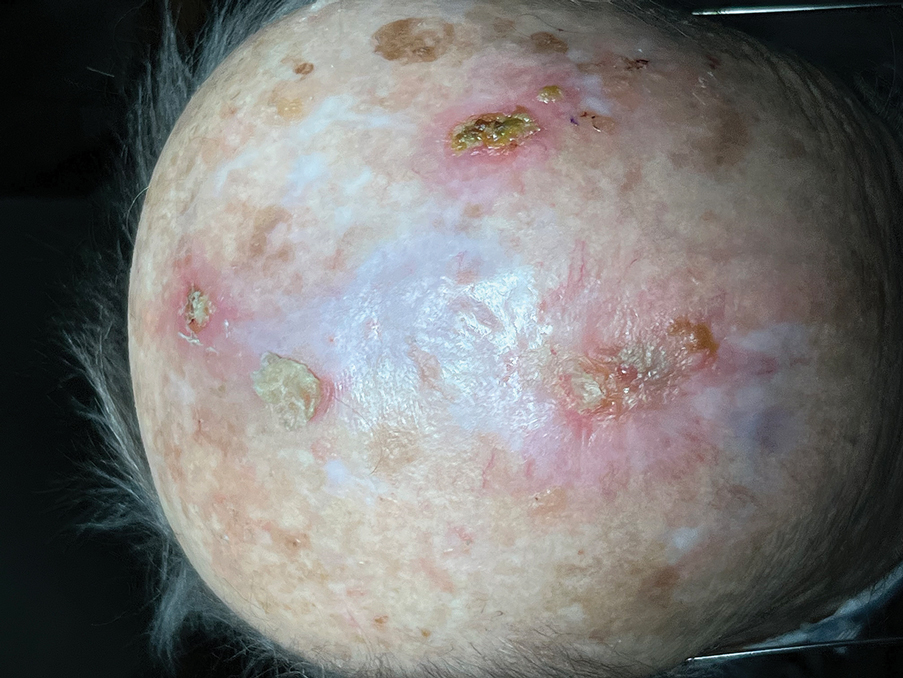
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).
Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.
Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
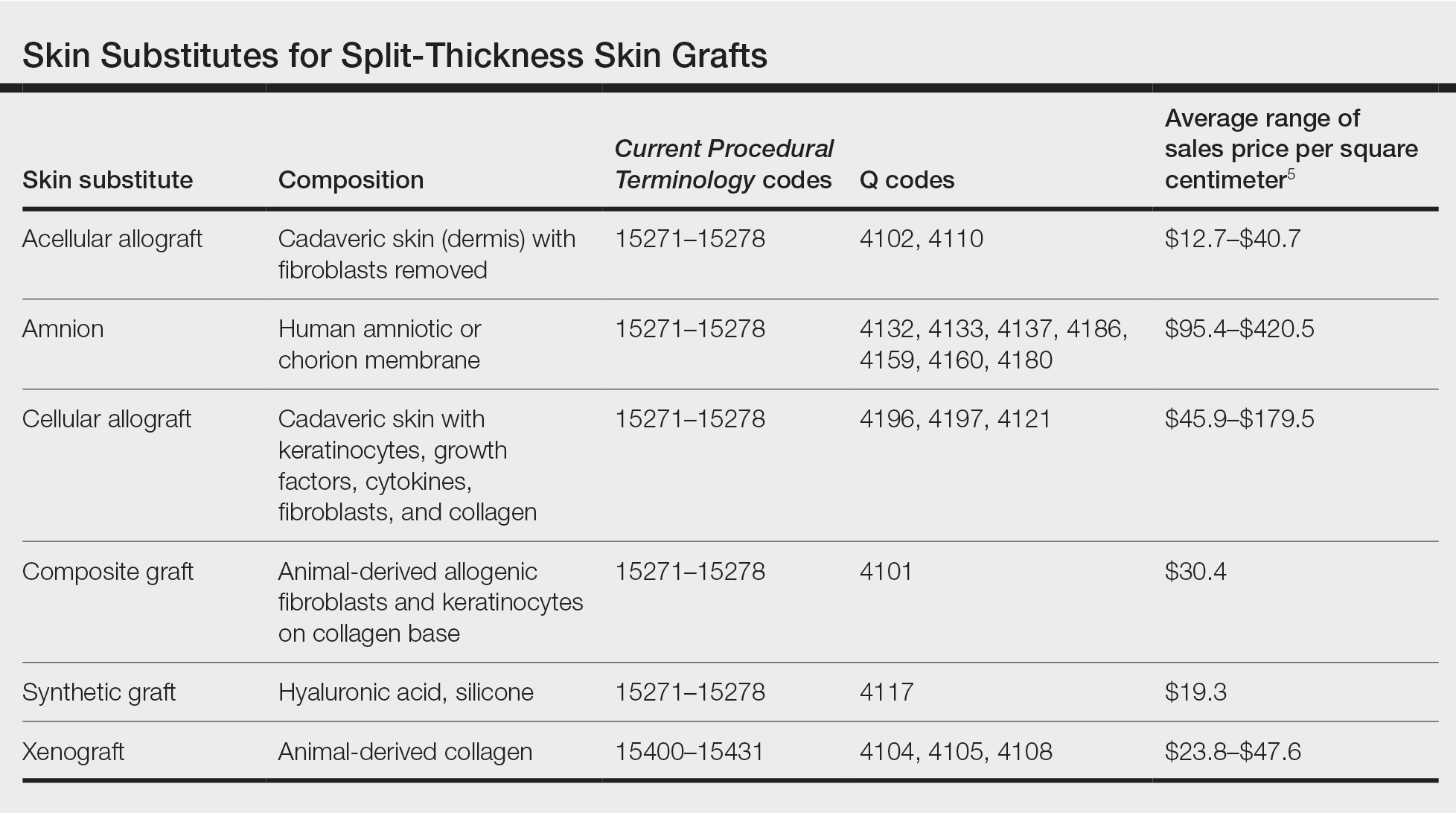
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).
Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
