User login
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
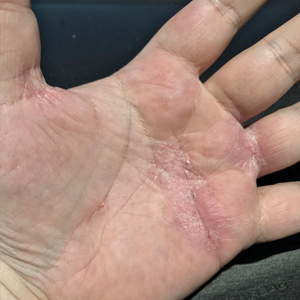
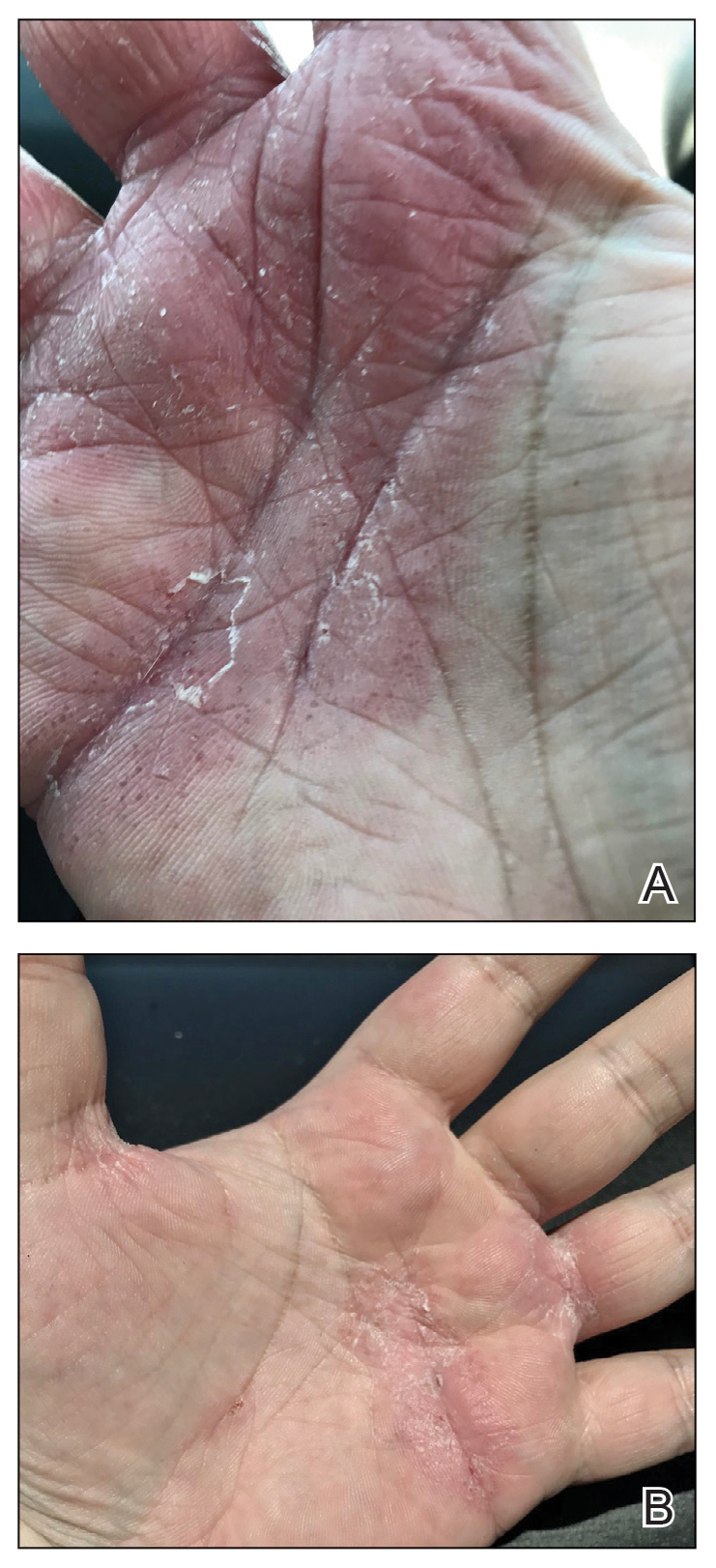
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2
Technique
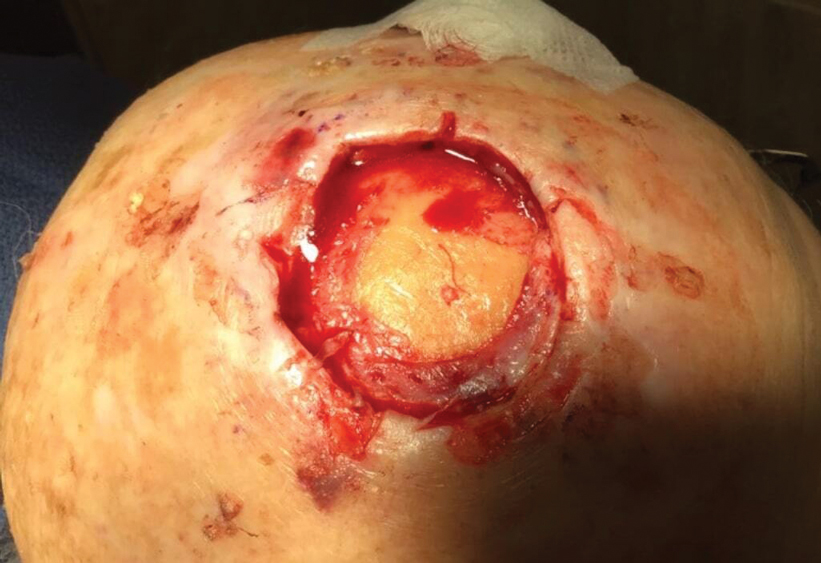
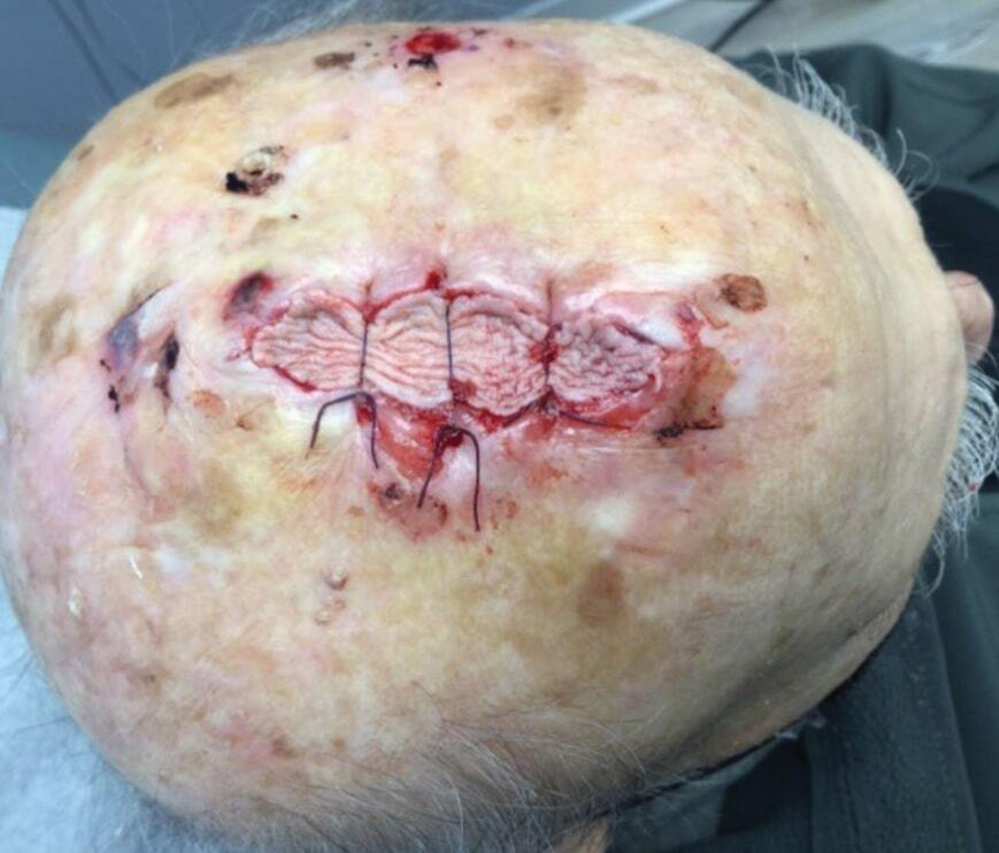
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).
Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.
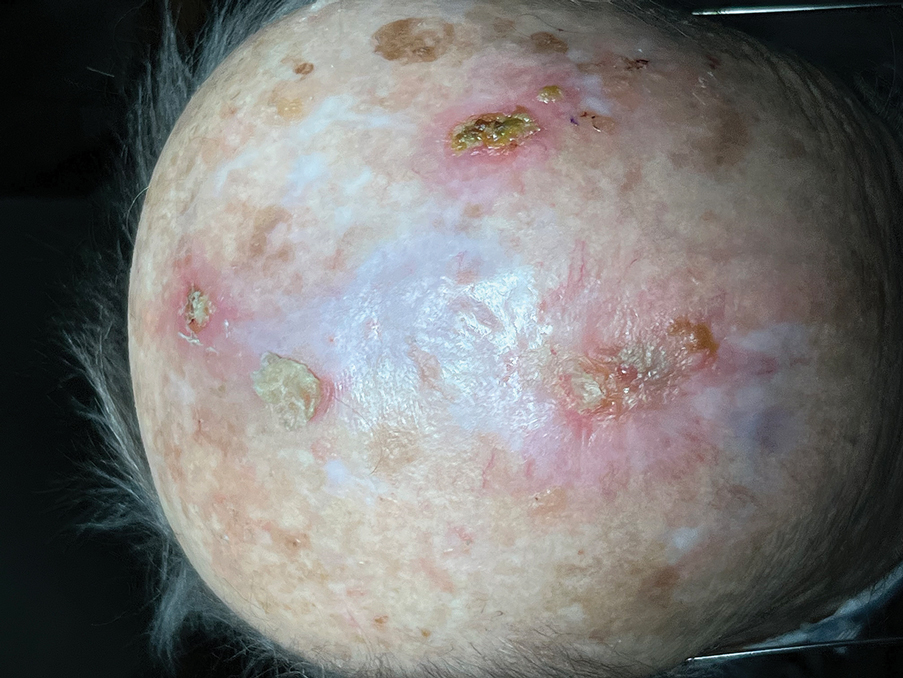
Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
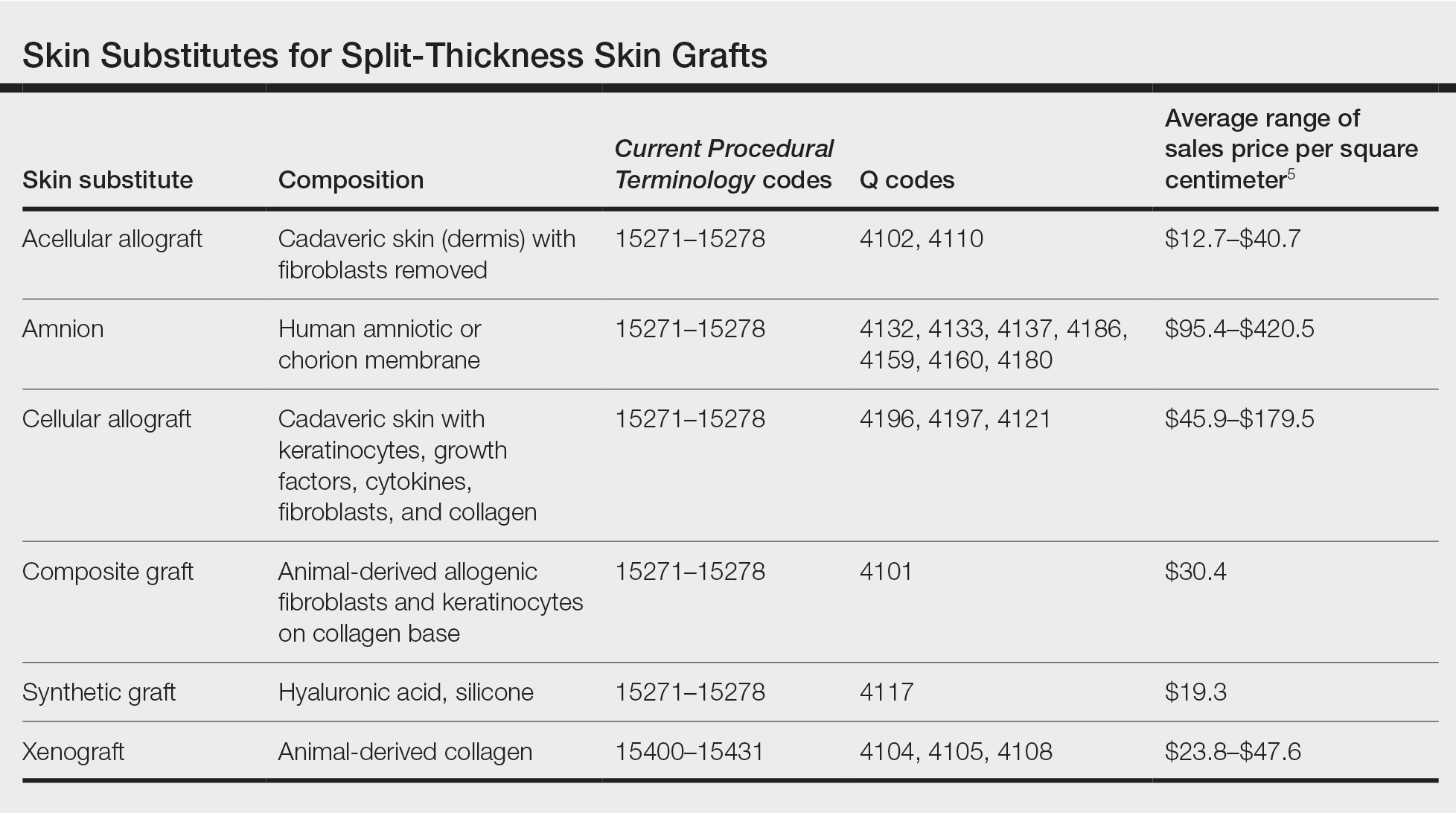
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).
Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Treatment of Angiosarcoma of the Head and Neck: A Systematic Review
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51
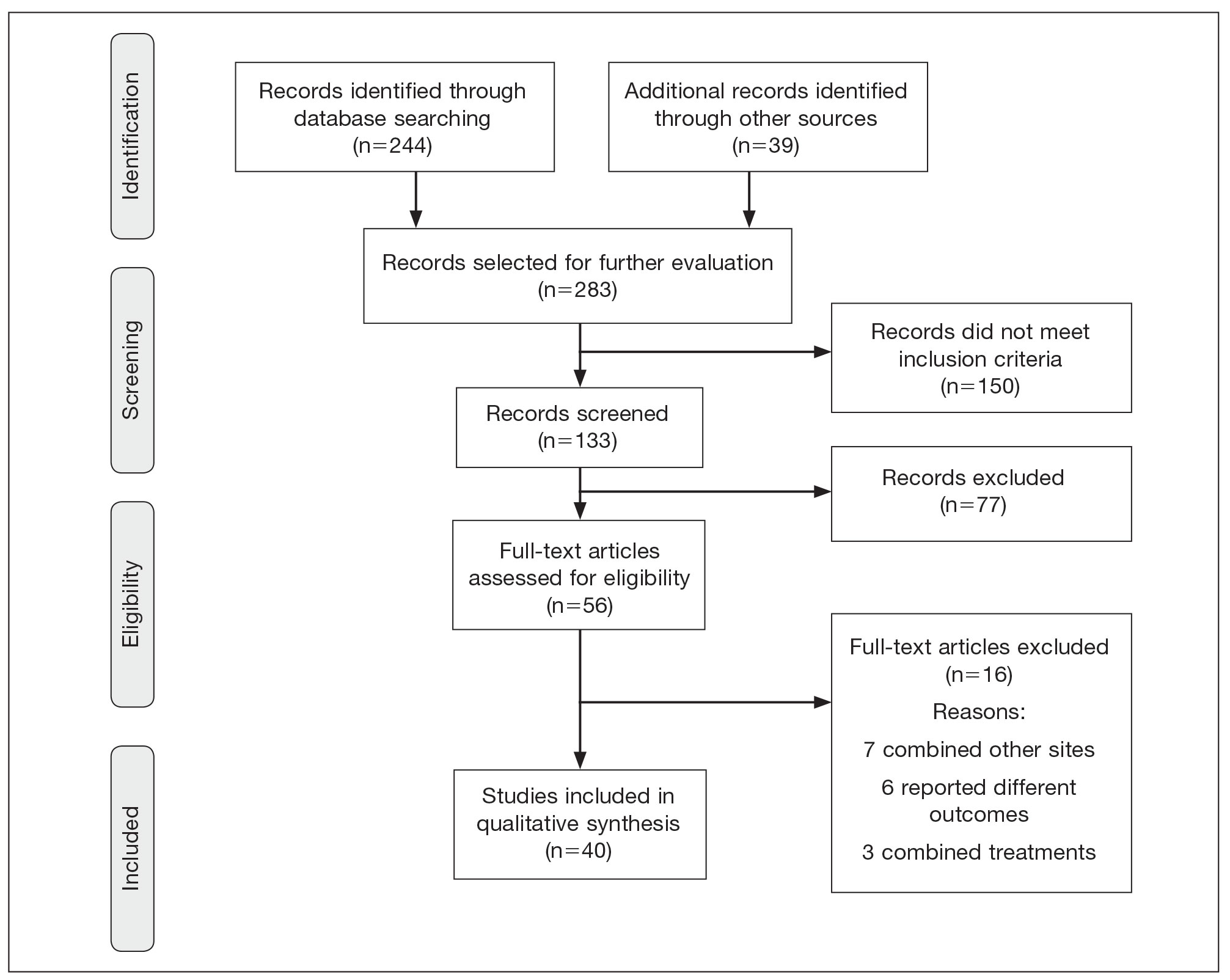
Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
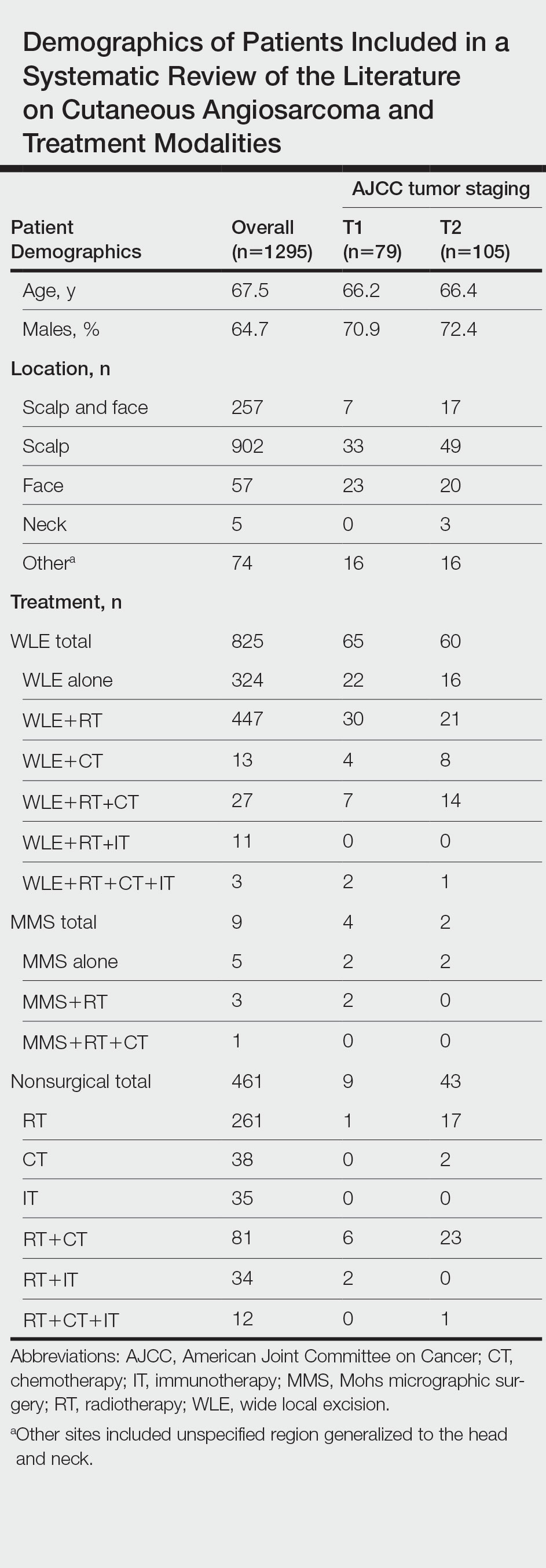
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).
Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).
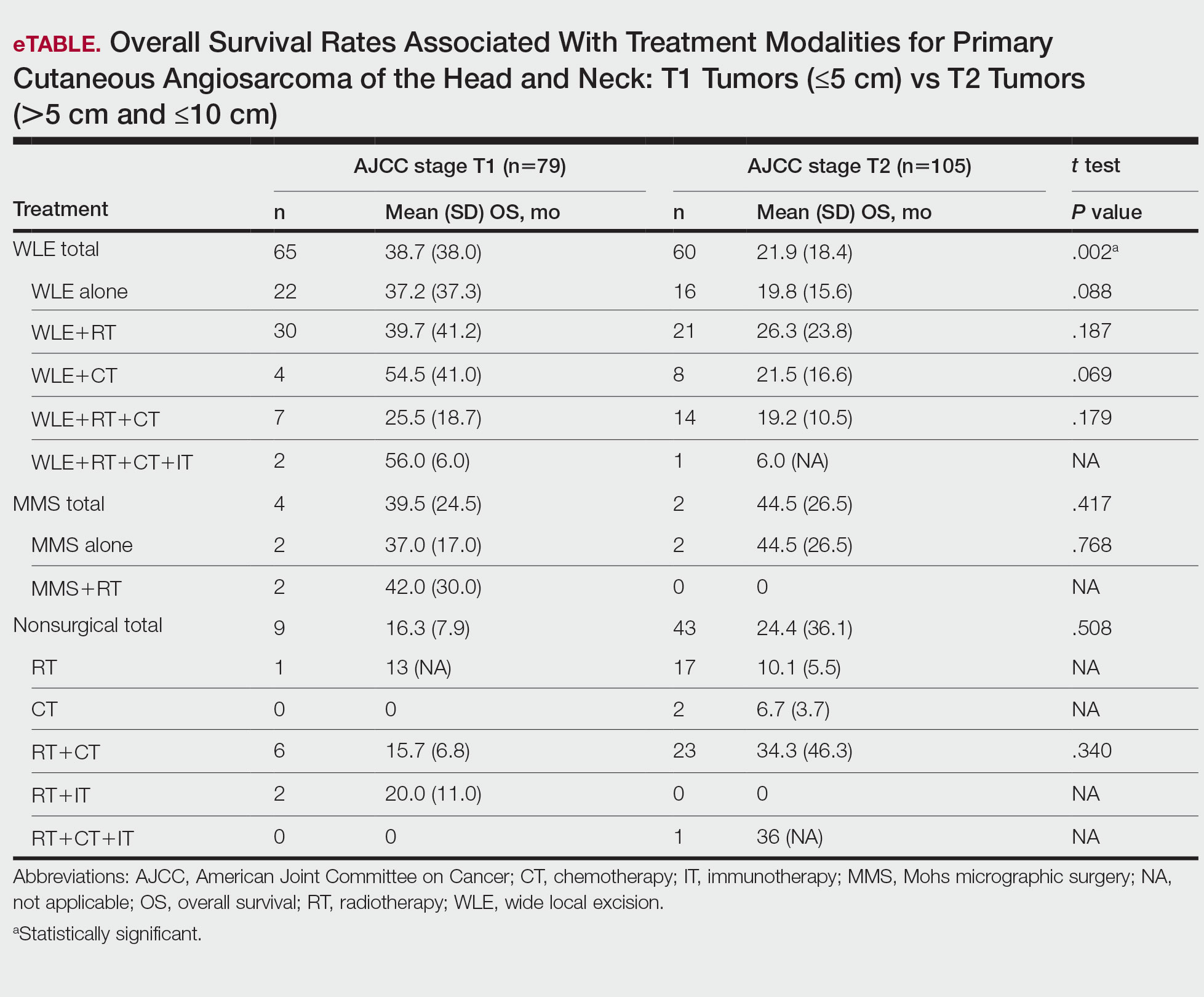
T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Practice Points
- Angiosarcoma is a rare tumor that is difficult to treat, with multiple treatment options being utilized.
- Within this systematic review, wide local excision (WLE) combined with radiotherapy (RT), chemotherapy, and immunotherapy, as well as Mohs micrographic surgery (MMS), offered the longest mean (SD) overall survival time.
- When clinicians are tasked with treating primary cutaneous angiosarcoma of the head and neck, they should consider MMS or WLE combined with RT.
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

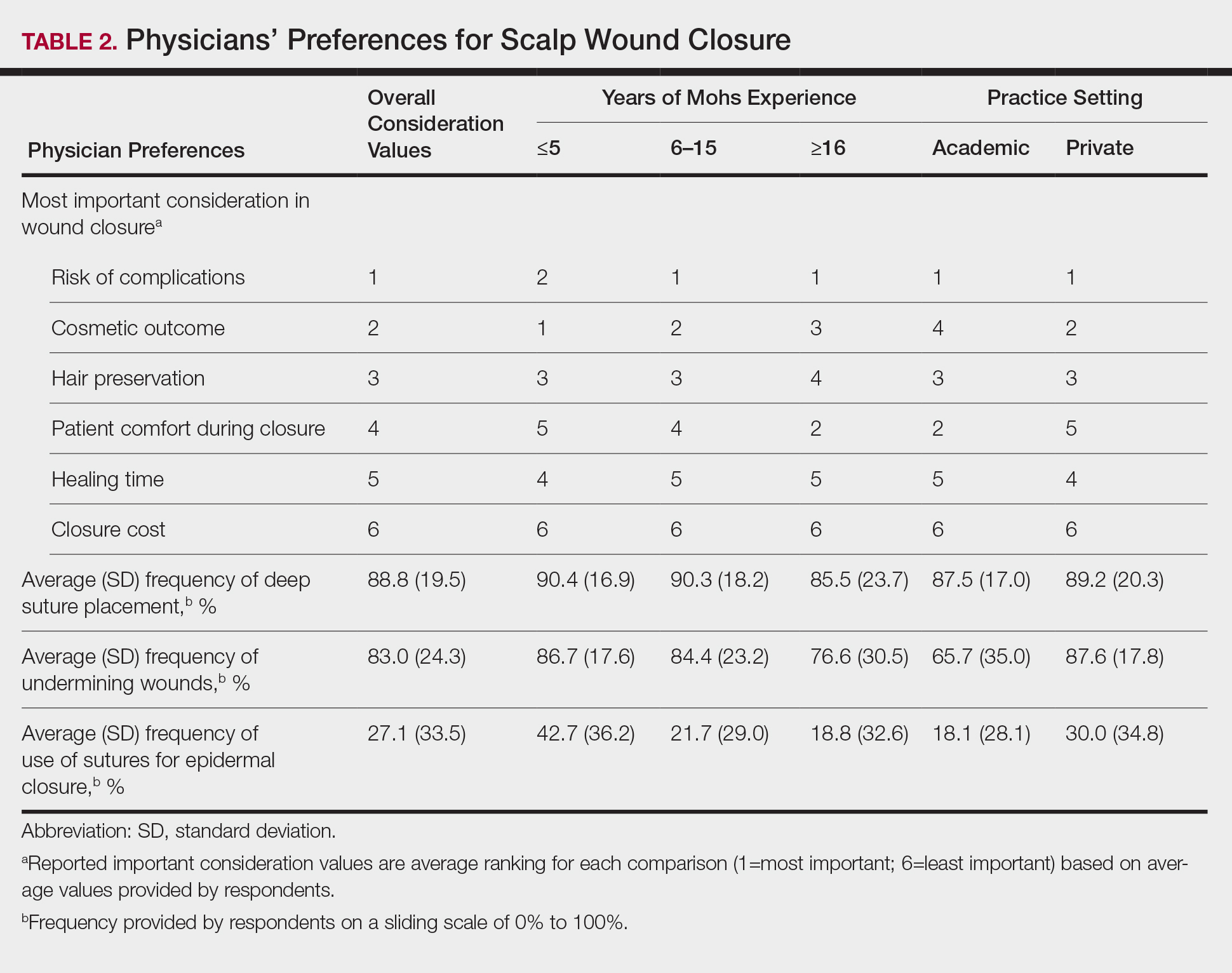
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
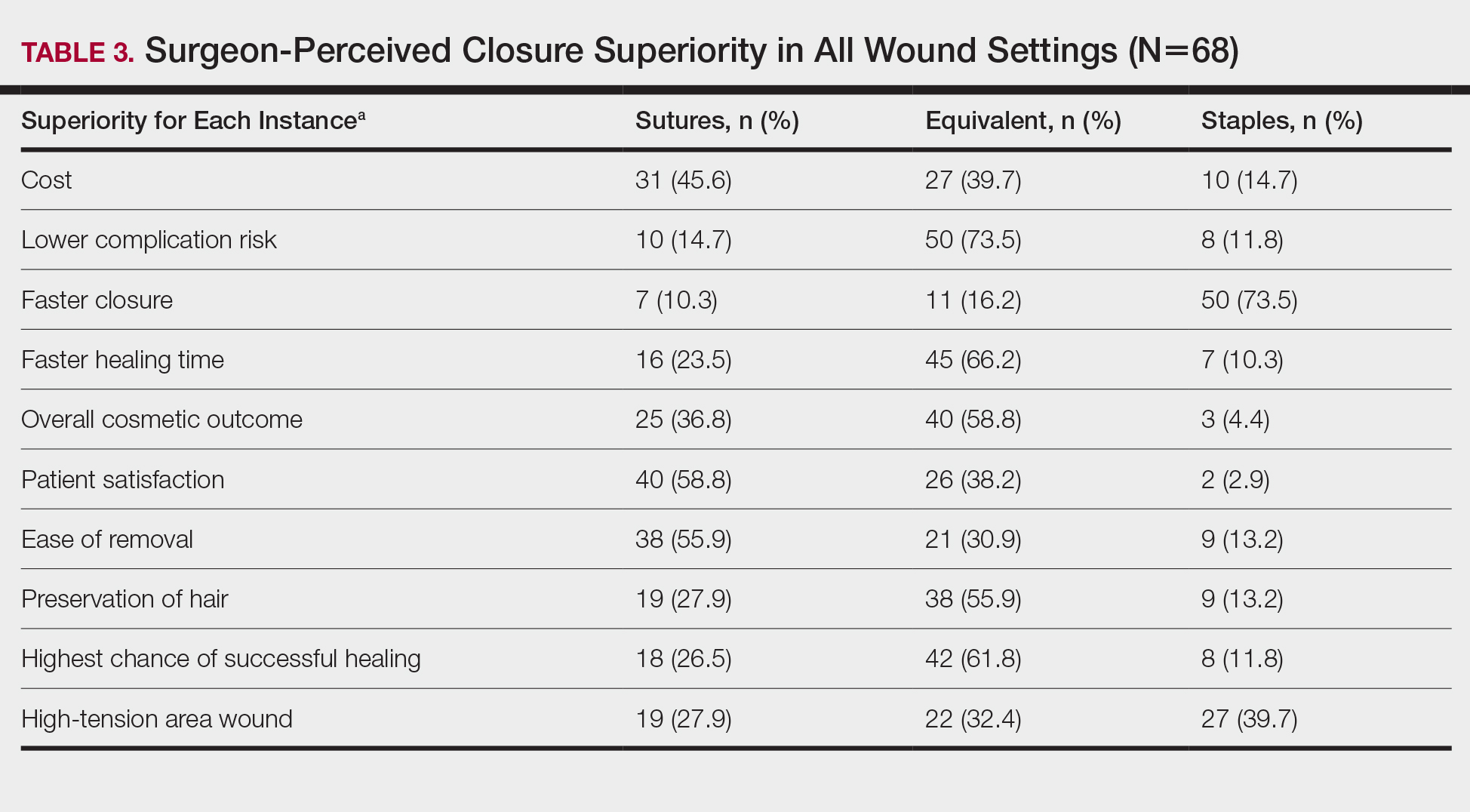
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
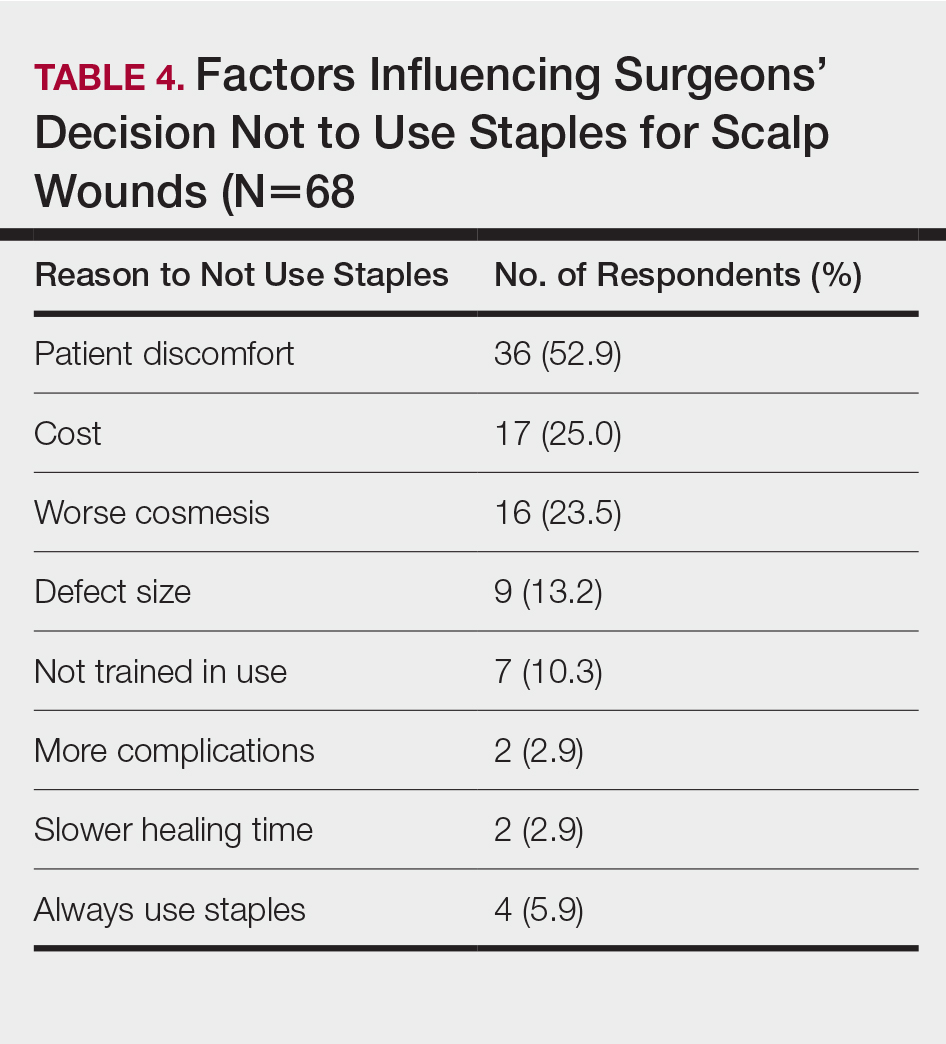
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.