User login
Is Frontal Fibrosing Alopecia Connected to Sunscreen Usage?
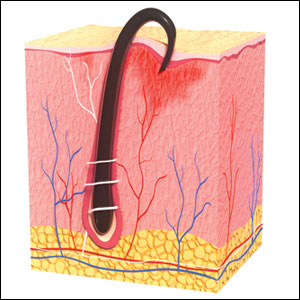
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.
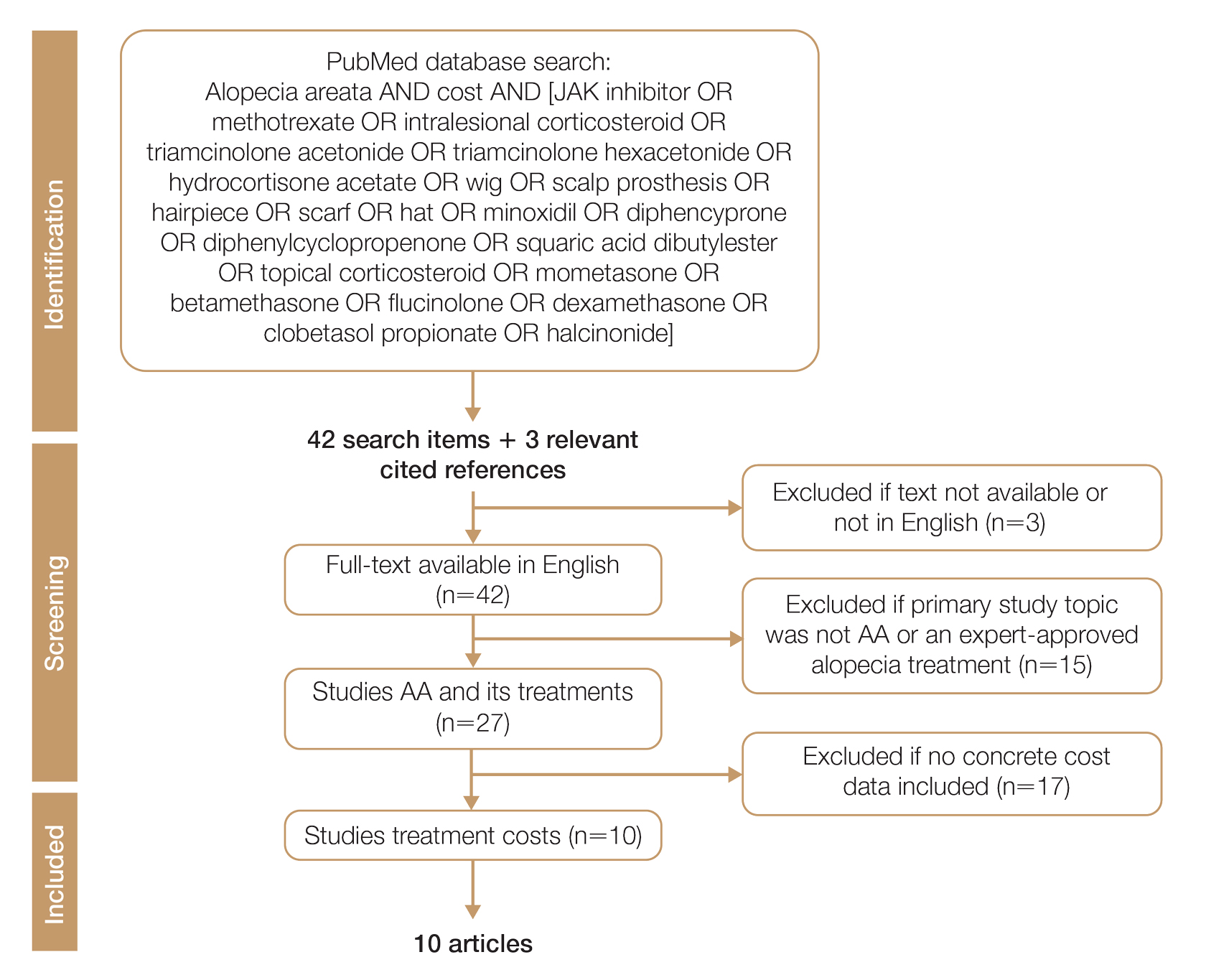
Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29
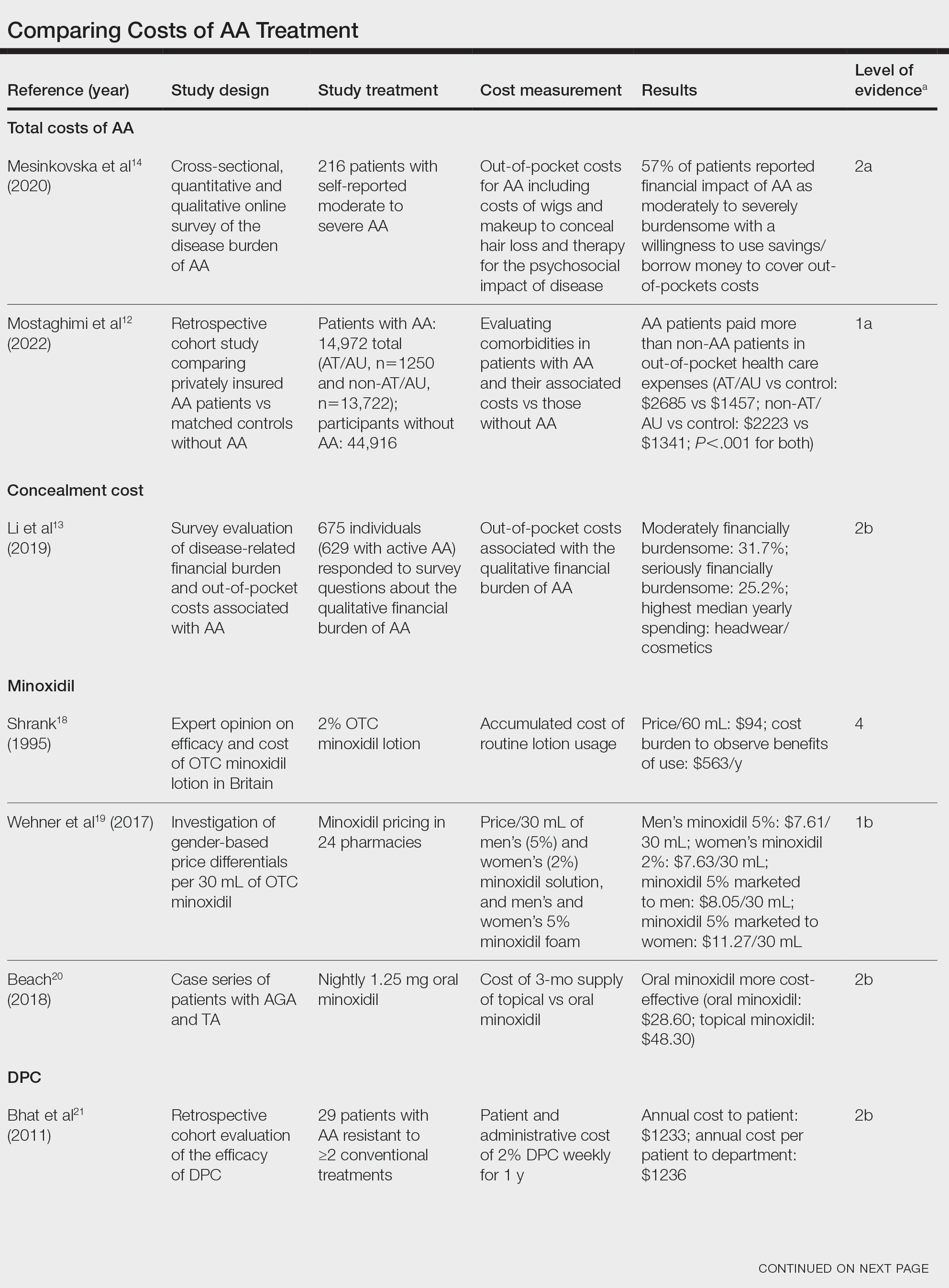
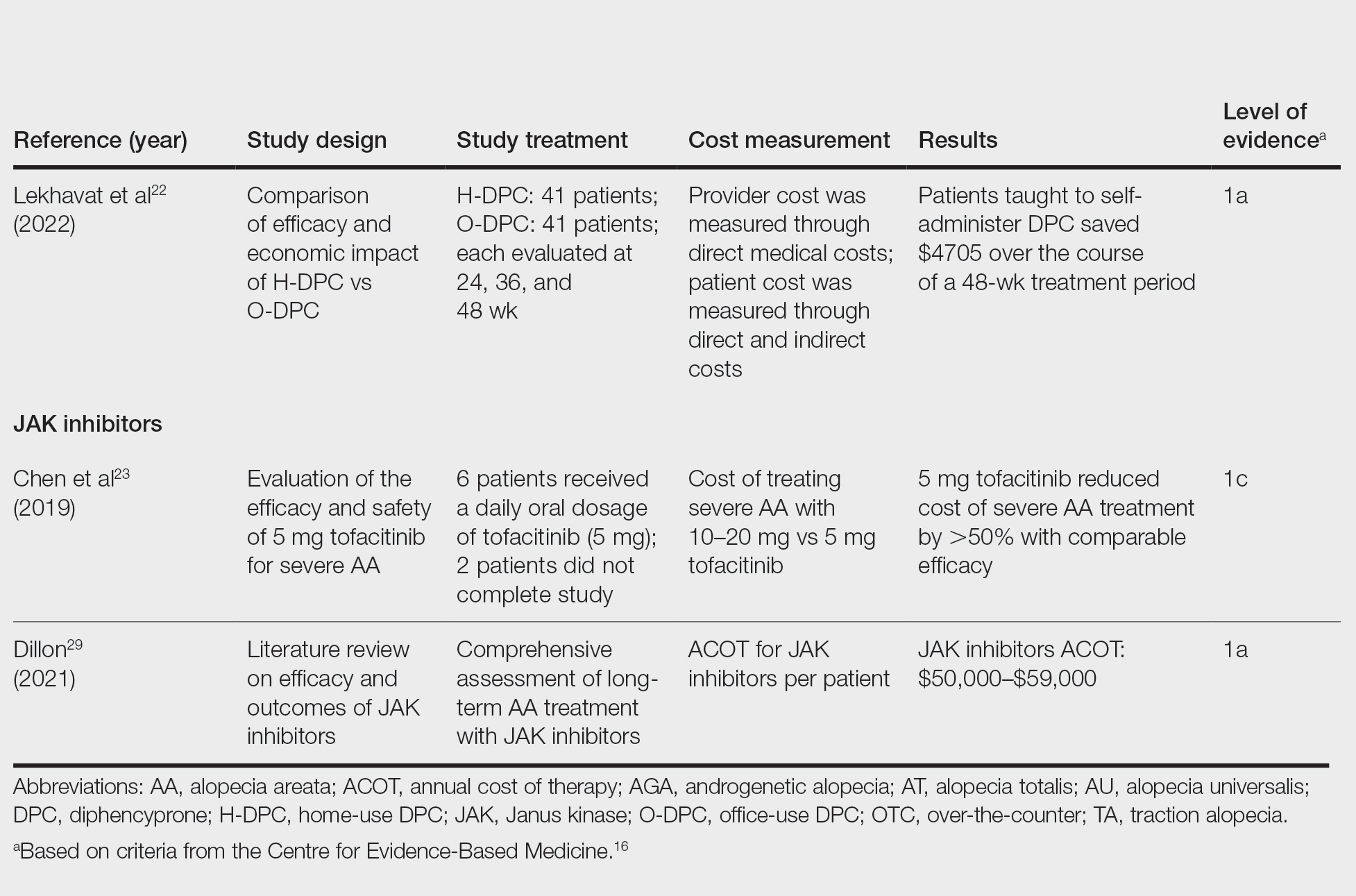
Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Practice Points
- Hair loss treatments and concealment techniques cost the average patient thousands of dollars. Much of this cost burden comes from items not covered by insurance.
- Providers should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective option.
- Self-administering diphencyprone at home is more cost- and time-effective than in-office diphencyprone administration and does not decrease efficacy.
New Razor Technology Improves Appearance and Quality of Life in Men With Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.
With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.
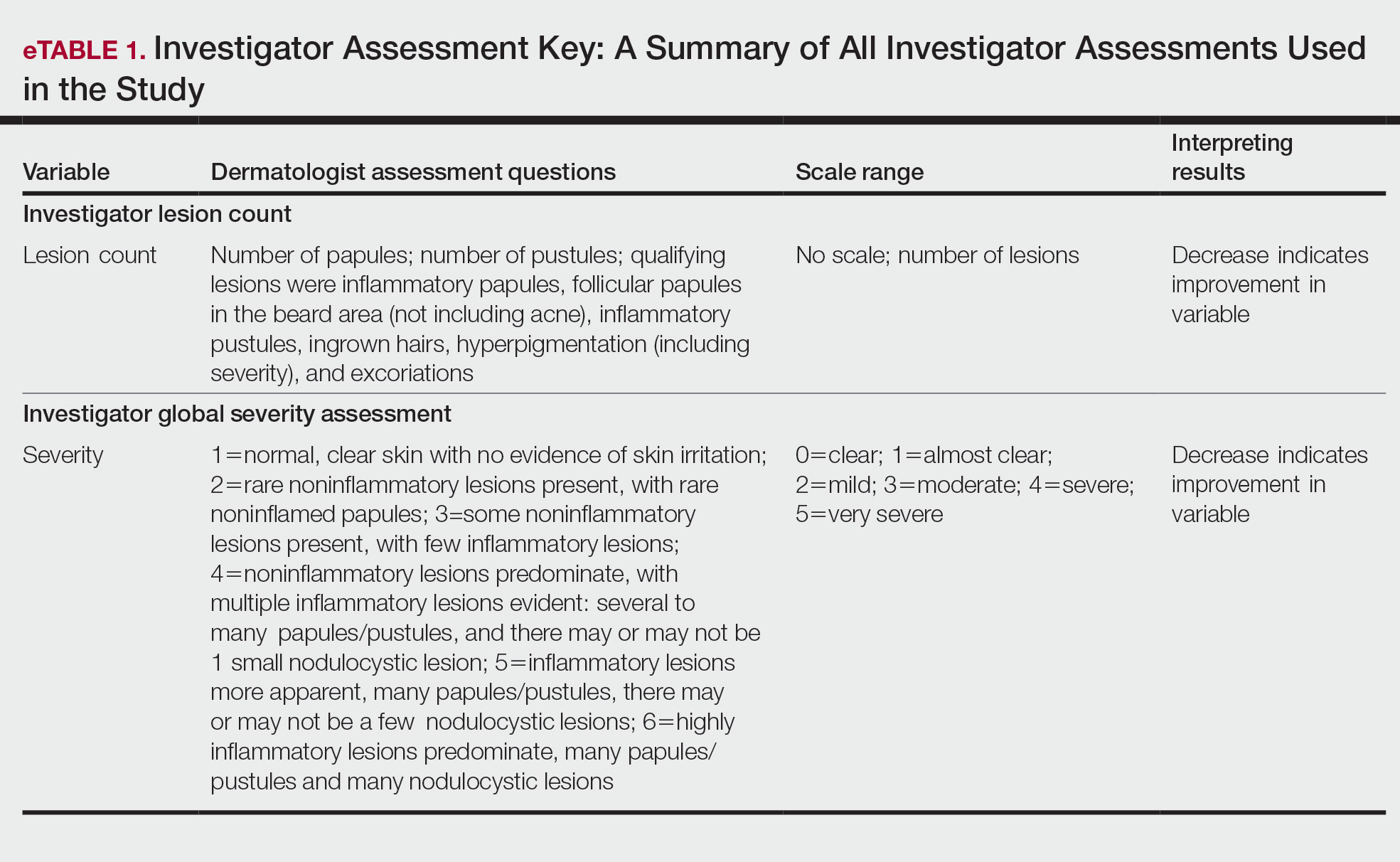
The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.
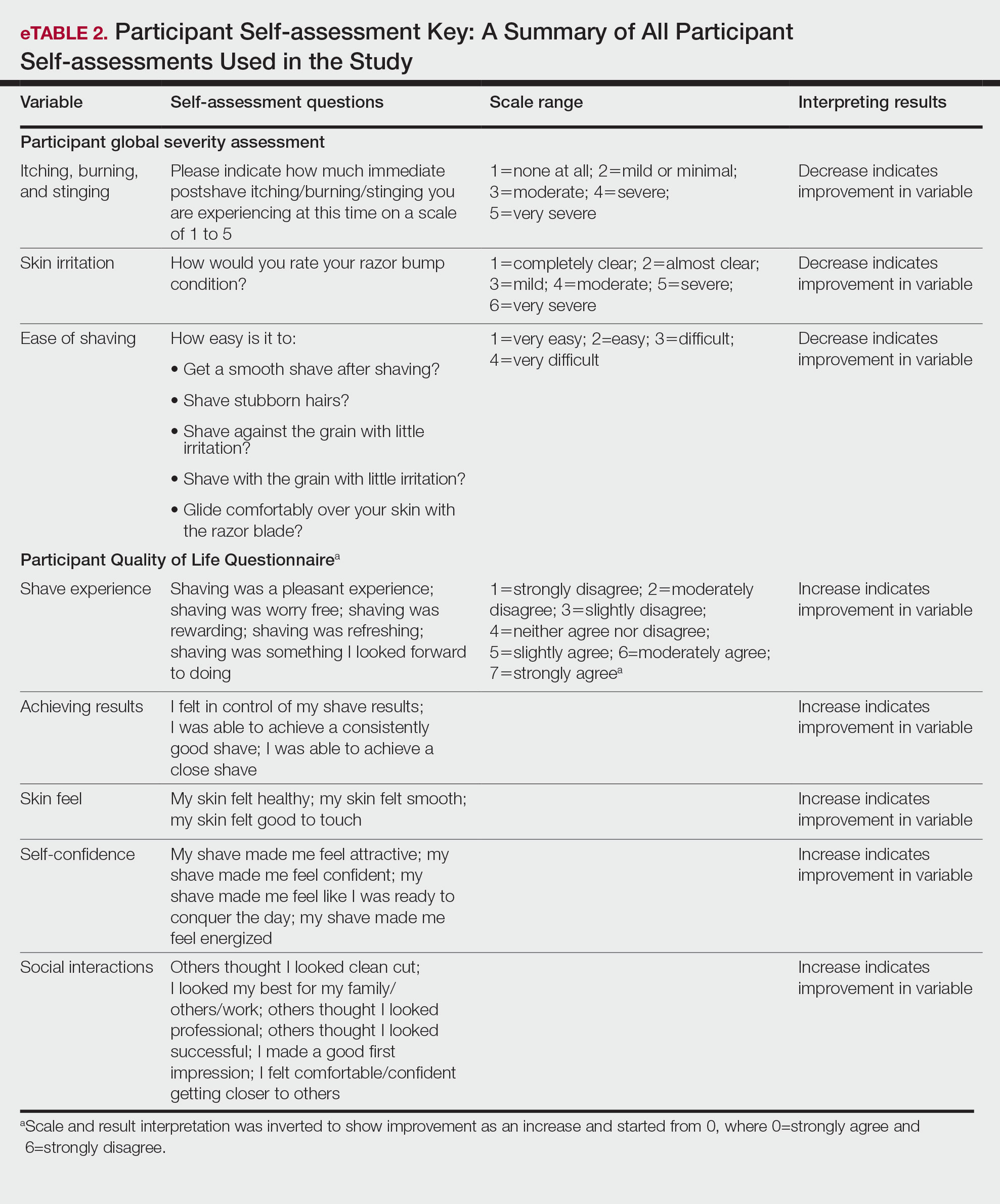
Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.
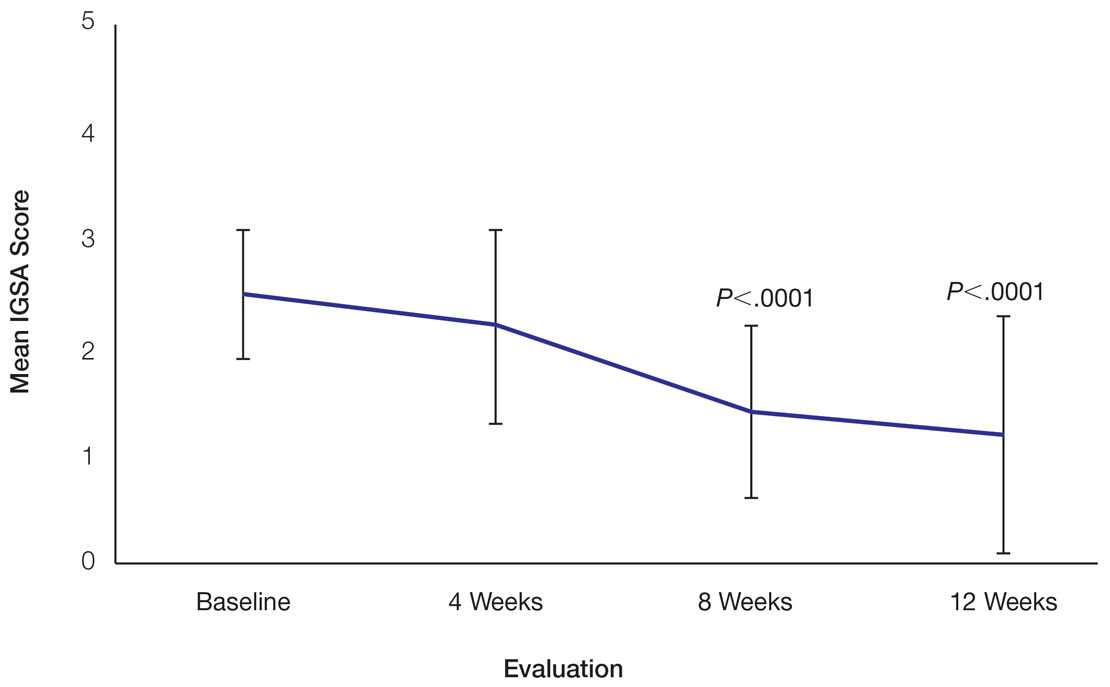
Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.
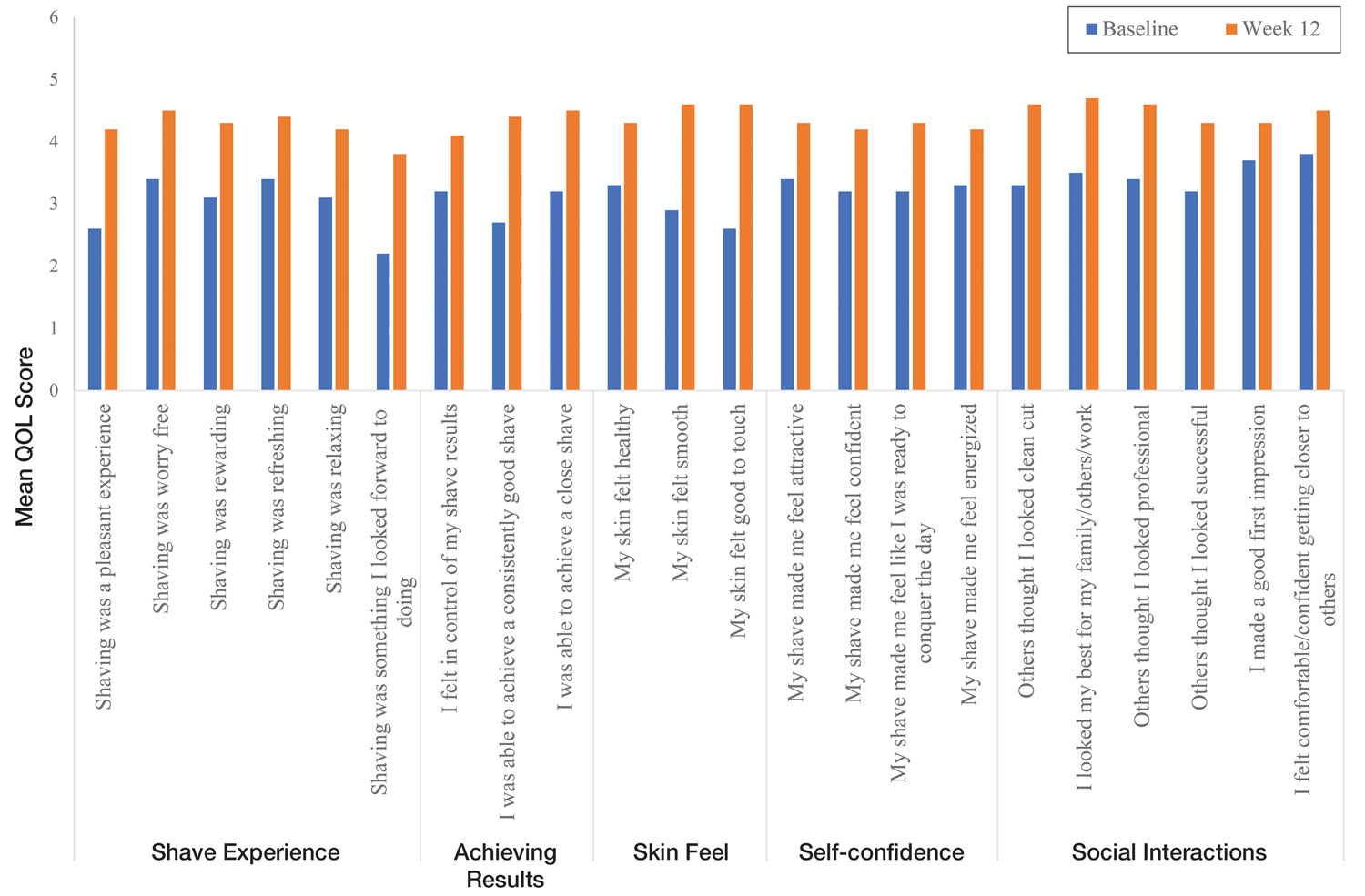
Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Practice Points
- Pseudofolliculitis barbae (PFB) is a common follicular inflammatory disorder associated with shaving, most commonly seen in men of African ancestry. It can be distressing and cause a substantial impact on quality of life (QOL).
- Frequent use of a novel razor technology designed specifically for men with PFB was found to improve skin appearance and QOL after 12 weeks vs baseline.
- This razor technology provides an alternative approach to help manage PFB for men who wish to or need to continue shaving.
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Diversity in Dermatology: A Society Devoted to Skin of Color
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
Practice Points
- The mission of the Skin of Color Society (SOCS) is to improve education of young dermatologists relevant to skin of color patients.
- Educational resources on many different diseases important to patients with skin of color are available to patients and providers on the SOCS website.