User login
Is Frontal Fibrosing Alopecia Connected to Sunscreen Usage?
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
Frontal fibrosing alopecia (FFA) has become increasingly common since it was first described in 1994.1 A positive correlation between FFA and the use of sunscreens was reported in an observational study.2 The geographic distribution of this association has spanned the United Kingdom (UK), Europe, and Asia, though data from the United States are lacking. Various international studies have demonstrated an association between FFA and sunscreen use, further exemplifying this stark contrast.
In the United Kingdom (UK), Aldoori et al2 found that women who used sunscreen at least twice weekly had 2 times the likelihood of developing FFA compared with women who did not use sunscreen regularly. Kidambi et al3 found similar results in UK men with FFA who had higher rates of primary sunscreen use and higher rates of at least twice-weekly use of facial moisturizer with unspecified sunscreen content.
These associations between FFA and sunscreen use are not unique to the UK. A study conducted in Spain identified a statistical association between FFA and use of facial sunscreen in women (odds ratio, 1.6 [95% CI, 1.06-2.41]) and men (odds ratio, 1.84 [95% CI, 1.04-3.23]).4 In Thailand, FFA was nearly twice as likely to be present in patients with regular sunscreen use compared to controls who did not apply sunscreen regularly.5 Interestingly, a Brazilian study showed no connection between sunscreen use and FFA. Instead, FFA was associated with hair straightening with formalin or use of facial soap orfacial moisturizer.6 An international systematic review of 1248 patients with FFA and 1459 controls determined that sunscreen users were 2.21 times more likely to develop FFA than their counterparts who did not use sunscreen regularly.7
Quite glaring is the lack of data from the United States, which could be used to compare FFA and sunscreen associations to other nations. It is possible that certain regions of the world such as the United States may not have an increased risk for FFA in sunscreen users due to other environmental factors, differing sunscreen application practices, or differing chemical ingredients. At the same time, many other countries cannot afford or lack access to sunscreens or facial moisturizers, which is an additional variable that may complicate this association. These populations need to be studied to determine whether they are as susceptible to FFA as those who use sunscreen regularly around the world.
Another underlying factor supporting this association is the inherent need for sunscreen use. For instance, research has shown that patients with FFA had higher rates of actinic skin damage, which could explain increased sunscreen use.8
To make more clear and distinct claims, further studies are needed in regions that are known to use sunscreen extensively (eg, United States) to compare with their European, Asian, and South American counterparts. Moreover, it also is important to study regions where sunscreen access is limited and whether there is FFA development in these populations.
Given the potential association between sunscreen use and FFA, dermatologists can take a cautious approach tailored to the patient by recommending noncomedogenic mineral sunscreens with zinc or titanium oxide, which are less irritating than chemical sunscreens. Avoidance of sunscreen application to the hairline and use of additional sun-protection methods such as broad-brimmed hats also should be emphasized.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens: a questionnaire study. Br J Dermatol. 2016;175:762-767.
- Kidambi AD, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with leave-on facial cosmetics and sunscreens. Br J Dermatol. 2020;175:61-67.
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Leecharoen W, Thanomkitti K, Thuangtong R, et al. Use of facial care products and frontal fibrosing alopecia: coincidence or true association? J Dermatol. 2021;48:1557-1563.
- Müller Ramos P, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.07
- Kam O, Na S, Guo W, et al. Frontal fibrosing alopecia and personal care product use: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315:2313-2331. doi:10.1007/s00403-023-02604-7
- Porriño-Bustamante ML, Montero-Vílchez T, Pinedo-Moraleda FJ, et al. Frontal fibrosing alopecia and sunscreen use: a cross-sectionalstudy of actinic damage. Acta Derm Venereol. Published online August 11, 2022. doi:10.2340/actadv.v102.306
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.
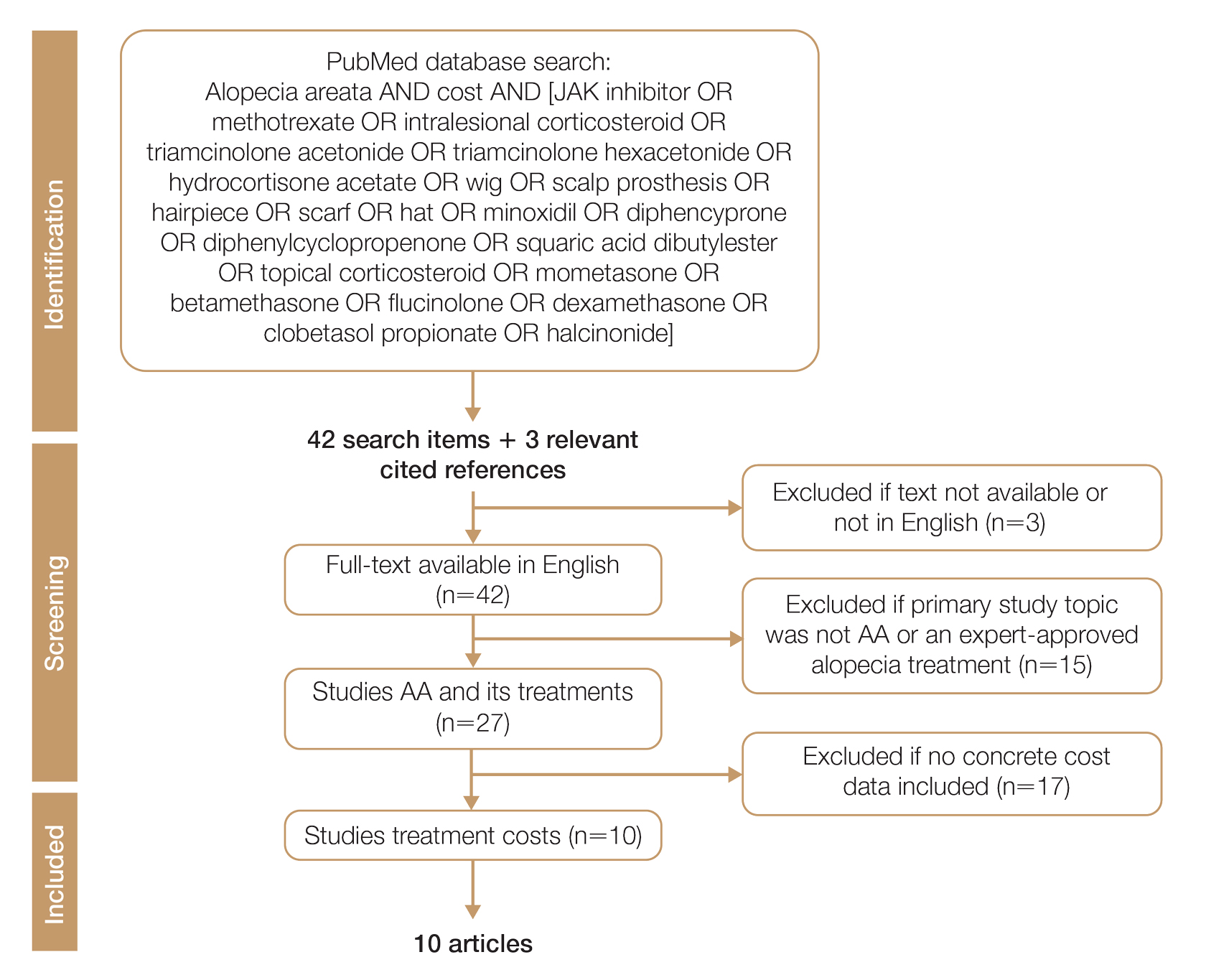
Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
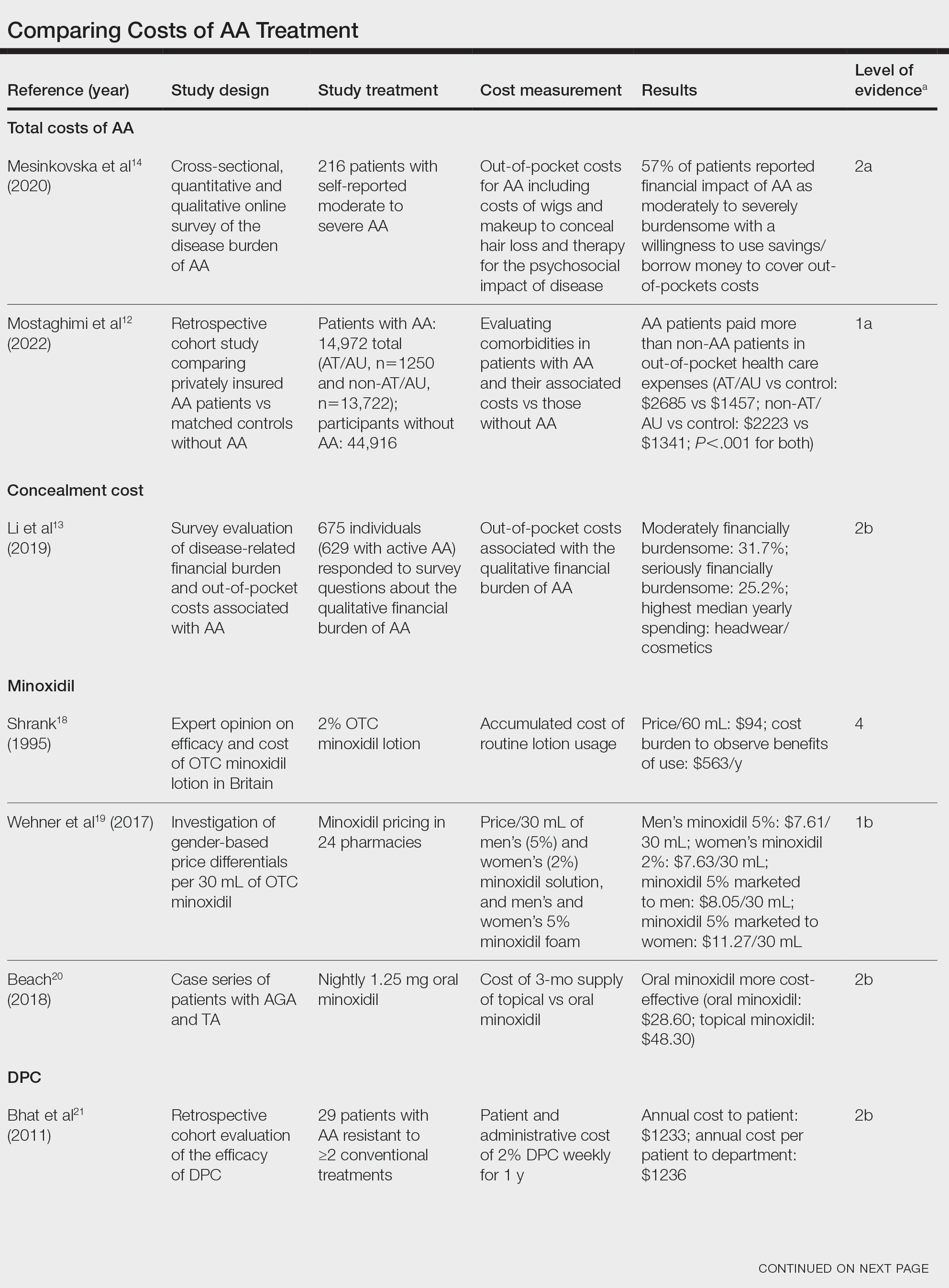
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
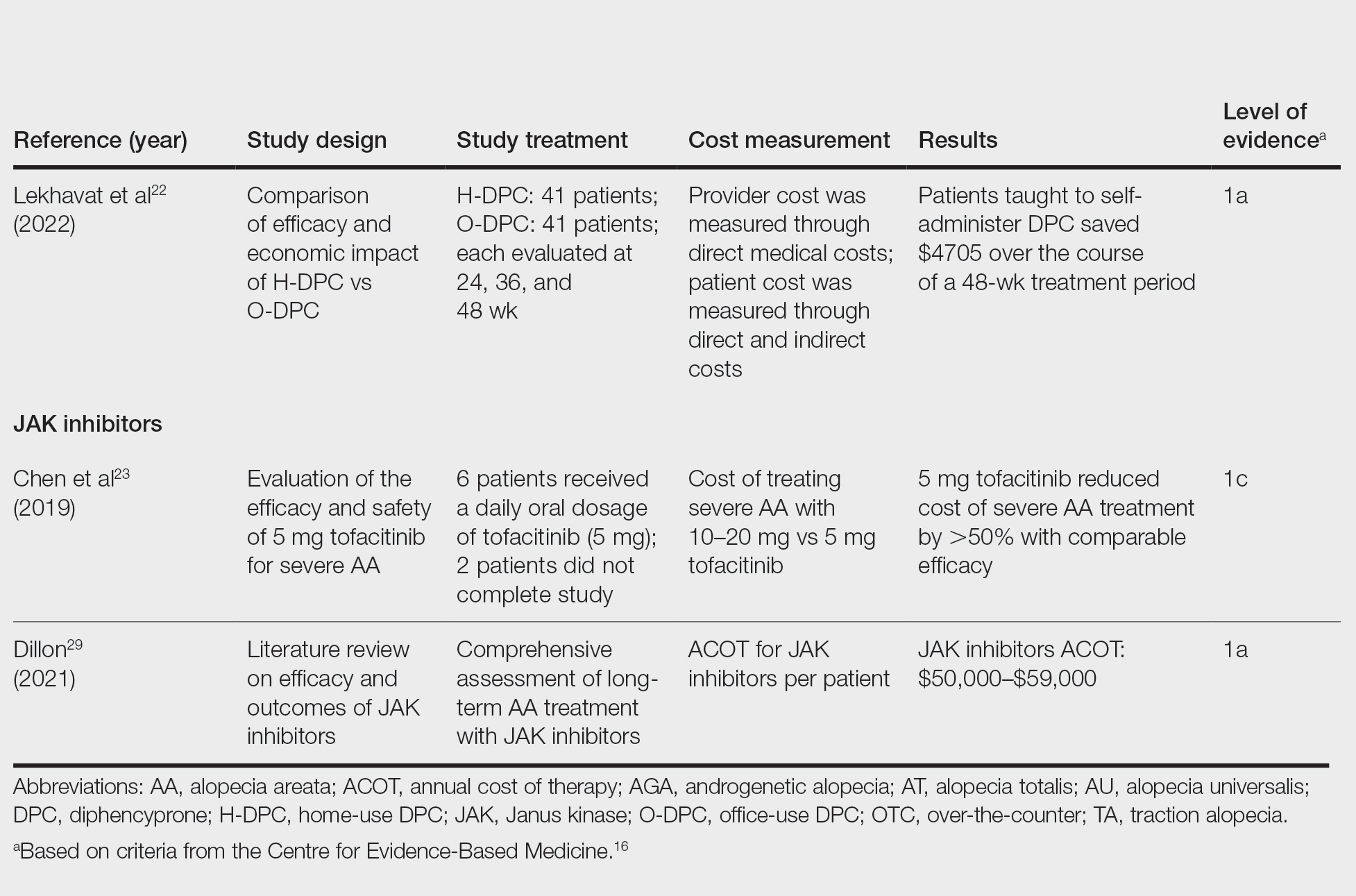
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29
Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Practice Points
- Hair loss treatments and concealment techniques cost the average patient thousands of dollars. Much of this cost burden comes from items not covered by insurance.
- Providers should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective option.
- Self-administering diphencyprone at home is more cost- and time-effective than in-office diphencyprone administration and does not decrease efficacy.
Alopecia Areata in Skin of Color Patients: New Considerations Sparked by the Approval of Baricitinib
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf

