User login
Sea Buckthorn
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
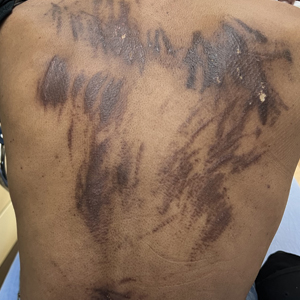
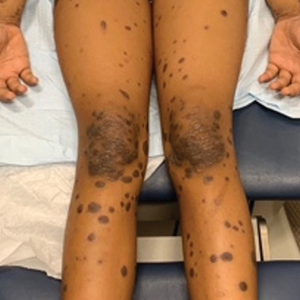
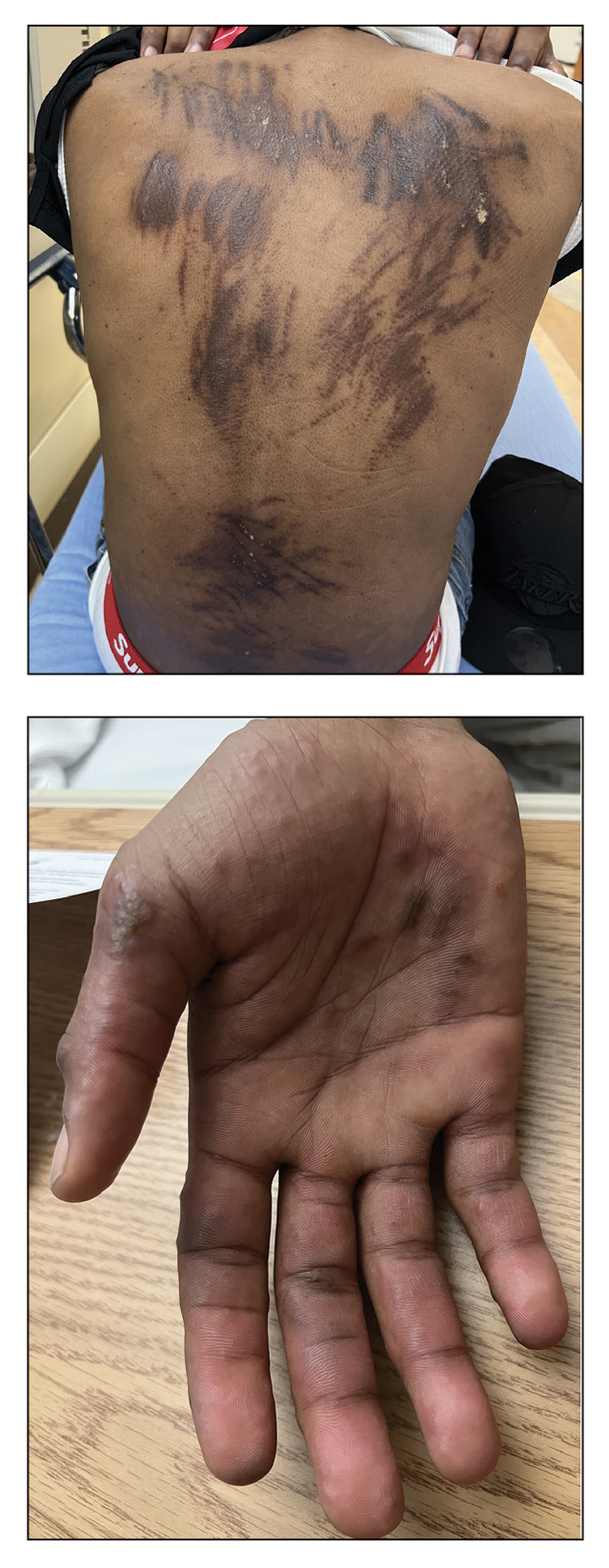
Eruption of Multiple Linear Hyperpigmented Plaques
THE DIAGNOSIS: Chemotherapy-Induced Flagellate Dermatitis
Based on the clinical presentation and temporal relation with chemotherapy, a diagnosis of bleomycininduced flagellate dermatitis (FD) was made, as bleomycin is the only chemotherapeutic agent from this regimen that has been linked with FD.1,2 Laboratory findings revealed eosinophilia, further supporting a druginduced dermatitis. The patient was treated with oral steroids and diphenhydramine to alleviate itching and discomfort. The chemotherapy was temporarily discontinued until symptomatic improvement was observed within 2 to 3 days.
Flagellate dermatitis is characterized by unique erythematous, linear, intermingled streaks of adjoining firm papules—often preceded by a prodrome of global pruritus—that eventually become hyperpigmented as the erythema subsides. The clinical manifestation of FD can be idiopathic; true/mechanical (dermatitis artefacta, abuse, sadomasochism); chemotherapy induced (peplomycin, trastuzumab, cisplatin, docetaxel, bendamustine); toxin induced (shiitake mushroom, cnidarian stings, Paederus insects); related to rheumatologic diseases (dermatomyositis, adult-onset Still disease), dermatographism, phytophotodermatitis, or poison ivy dermatitis; or induced by chikungunya fever.1
The term flagellate originates from the Latin word flagellum, which pertains to the distinctive whiplike pattern. It was first described by Moulin et al3 in 1970 in reference to bleomycin-induced linear hyperpigmentation. Bleomycin, a glycopeptide antibiotic derived from Streptomyces verticillus, is used to treat Hodgkin lymphoma, squamous cell carcinoma, and germ cell tumors. The worldwide incidence of bleomycin-induced FD is 8% to 22% and commonly is associated with a cumulative dose greater than 100 U.2 Clinical presentation is variable in terms of onset, distribution, and morphology of the eruption and could be independent of dose, route of administration, or type of malignancy being treated. The flagellate rash commonly involves the trunk, arms, and legs; can develop within hours to 6 months of starting bleomycin therapy; often is preceded by generalized itching; and eventually heals with hyperpigmentation.
Possible mechanisms of bleomycin-induced FD include localized melanogenesis, inflammatory pigmentary incontinence, alterations to normal pigmentation patterns, cytotoxic effects of the drug itself, minor trauma/ scratching leading to increased blood flow and causing local accumulation of bleomycin, heat recall, and reduced epidermal turnover leading to extended interaction between keratinocytes and melanocytes.2 Heat exposure can act as a trigger for bleomycin-induced skin rash recall even months after the treatment is stopped.
Apart from discontinuing the drug, there is no specific treatment available for bleomycin-induced FD. The primary objective of treatment is to alleviate pruritus, which often involves the use of topical or systemic corticosteroids and oral antihistamines. The duration of treatment depends on the patient’s clinical response. Once treatment is discontinued, FD typically resolves within 6 to 8 months. However, there can be a permanent postinflammatory hyperpigmentation in the affected area.4 Although there is a concern for increased mortality after postponement of chemotherapy,5 the decision to proceed with or discontinue the chemotherapy regimen necessitates a comprehensive interdisciplinary discussion and a meticulous assessment of the risks and benefits that is customized to each individual patient. Flagellate dermatitis can reoccur with bleomycin re-exposure; a combined approach of proactive topical and systemic steroid treatment seems to diminish the likelihood of FD recurrence.5
Our case underscores the importance of recognizing, detecting, and managing FD promptly in individuals undergoing bleomycin-based chemotherapy. Medical professionals should familiarize themselves with this distinct adverse effect linked to bleomycin, enabling prompt discontinuation if necessary, and educate patients about the condition’s typically temporary nature, thereby alleviating their concerns.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol. 2014;80:149-152.
- Ziemer M, Goetze S, Juhasz K, et al. Flagellate dermatitis as a bleomycinspecific adverse effect of cytostatic therapy: a clinical-histopathologic correlation. Am J Clin Dermatol. 2011;12:68-76. doi:10.2165/11537080-000000000-00000
- Moulin G, Fière B, Beyvin A. Cutaneous pigmentation caused by bleomycin. Article in French. Bull Soc Fr Dermatol Syphiligr. 1970;77:293-296.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503. doi:10.4103/0973-1482.119358
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi:10.1136/bmj.m4087
THE DIAGNOSIS: Chemotherapy-Induced Flagellate Dermatitis
Based on the clinical presentation and temporal relation with chemotherapy, a diagnosis of bleomycininduced flagellate dermatitis (FD) was made, as bleomycin is the only chemotherapeutic agent from this regimen that has been linked with FD.1,2 Laboratory findings revealed eosinophilia, further supporting a druginduced dermatitis. The patient was treated with oral steroids and diphenhydramine to alleviate itching and discomfort. The chemotherapy was temporarily discontinued until symptomatic improvement was observed within 2 to 3 days.
Flagellate dermatitis is characterized by unique erythematous, linear, intermingled streaks of adjoining firm papules—often preceded by a prodrome of global pruritus—that eventually become hyperpigmented as the erythema subsides. The clinical manifestation of FD can be idiopathic; true/mechanical (dermatitis artefacta, abuse, sadomasochism); chemotherapy induced (peplomycin, trastuzumab, cisplatin, docetaxel, bendamustine); toxin induced (shiitake mushroom, cnidarian stings, Paederus insects); related to rheumatologic diseases (dermatomyositis, adult-onset Still disease), dermatographism, phytophotodermatitis, or poison ivy dermatitis; or induced by chikungunya fever.1
The term flagellate originates from the Latin word flagellum, which pertains to the distinctive whiplike pattern. It was first described by Moulin et al3 in 1970 in reference to bleomycin-induced linear hyperpigmentation. Bleomycin, a glycopeptide antibiotic derived from Streptomyces verticillus, is used to treat Hodgkin lymphoma, squamous cell carcinoma, and germ cell tumors. The worldwide incidence of bleomycin-induced FD is 8% to 22% and commonly is associated with a cumulative dose greater than 100 U.2 Clinical presentation is variable in terms of onset, distribution, and morphology of the eruption and could be independent of dose, route of administration, or type of malignancy being treated. The flagellate rash commonly involves the trunk, arms, and legs; can develop within hours to 6 months of starting bleomycin therapy; often is preceded by generalized itching; and eventually heals with hyperpigmentation.
Possible mechanisms of bleomycin-induced FD include localized melanogenesis, inflammatory pigmentary incontinence, alterations to normal pigmentation patterns, cytotoxic effects of the drug itself, minor trauma/ scratching leading to increased blood flow and causing local accumulation of bleomycin, heat recall, and reduced epidermal turnover leading to extended interaction between keratinocytes and melanocytes.2 Heat exposure can act as a trigger for bleomycin-induced skin rash recall even months after the treatment is stopped.
Apart from discontinuing the drug, there is no specific treatment available for bleomycin-induced FD. The primary objective of treatment is to alleviate pruritus, which often involves the use of topical or systemic corticosteroids and oral antihistamines. The duration of treatment depends on the patient’s clinical response. Once treatment is discontinued, FD typically resolves within 6 to 8 months. However, there can be a permanent postinflammatory hyperpigmentation in the affected area.4 Although there is a concern for increased mortality after postponement of chemotherapy,5 the decision to proceed with or discontinue the chemotherapy regimen necessitates a comprehensive interdisciplinary discussion and a meticulous assessment of the risks and benefits that is customized to each individual patient. Flagellate dermatitis can reoccur with bleomycin re-exposure; a combined approach of proactive topical and systemic steroid treatment seems to diminish the likelihood of FD recurrence.5
Our case underscores the importance of recognizing, detecting, and managing FD promptly in individuals undergoing bleomycin-based chemotherapy. Medical professionals should familiarize themselves with this distinct adverse effect linked to bleomycin, enabling prompt discontinuation if necessary, and educate patients about the condition’s typically temporary nature, thereby alleviating their concerns.
THE DIAGNOSIS: Chemotherapy-Induced Flagellate Dermatitis
Based on the clinical presentation and temporal relation with chemotherapy, a diagnosis of bleomycininduced flagellate dermatitis (FD) was made, as bleomycin is the only chemotherapeutic agent from this regimen that has been linked with FD.1,2 Laboratory findings revealed eosinophilia, further supporting a druginduced dermatitis. The patient was treated with oral steroids and diphenhydramine to alleviate itching and discomfort. The chemotherapy was temporarily discontinued until symptomatic improvement was observed within 2 to 3 days.
Flagellate dermatitis is characterized by unique erythematous, linear, intermingled streaks of adjoining firm papules—often preceded by a prodrome of global pruritus—that eventually become hyperpigmented as the erythema subsides. The clinical manifestation of FD can be idiopathic; true/mechanical (dermatitis artefacta, abuse, sadomasochism); chemotherapy induced (peplomycin, trastuzumab, cisplatin, docetaxel, bendamustine); toxin induced (shiitake mushroom, cnidarian stings, Paederus insects); related to rheumatologic diseases (dermatomyositis, adult-onset Still disease), dermatographism, phytophotodermatitis, or poison ivy dermatitis; or induced by chikungunya fever.1
The term flagellate originates from the Latin word flagellum, which pertains to the distinctive whiplike pattern. It was first described by Moulin et al3 in 1970 in reference to bleomycin-induced linear hyperpigmentation. Bleomycin, a glycopeptide antibiotic derived from Streptomyces verticillus, is used to treat Hodgkin lymphoma, squamous cell carcinoma, and germ cell tumors. The worldwide incidence of bleomycin-induced FD is 8% to 22% and commonly is associated with a cumulative dose greater than 100 U.2 Clinical presentation is variable in terms of onset, distribution, and morphology of the eruption and could be independent of dose, route of administration, or type of malignancy being treated. The flagellate rash commonly involves the trunk, arms, and legs; can develop within hours to 6 months of starting bleomycin therapy; often is preceded by generalized itching; and eventually heals with hyperpigmentation.
Possible mechanisms of bleomycin-induced FD include localized melanogenesis, inflammatory pigmentary incontinence, alterations to normal pigmentation patterns, cytotoxic effects of the drug itself, minor trauma/ scratching leading to increased blood flow and causing local accumulation of bleomycin, heat recall, and reduced epidermal turnover leading to extended interaction between keratinocytes and melanocytes.2 Heat exposure can act as a trigger for bleomycin-induced skin rash recall even months after the treatment is stopped.
Apart from discontinuing the drug, there is no specific treatment available for bleomycin-induced FD. The primary objective of treatment is to alleviate pruritus, which often involves the use of topical or systemic corticosteroids and oral antihistamines. The duration of treatment depends on the patient’s clinical response. Once treatment is discontinued, FD typically resolves within 6 to 8 months. However, there can be a permanent postinflammatory hyperpigmentation in the affected area.4 Although there is a concern for increased mortality after postponement of chemotherapy,5 the decision to proceed with or discontinue the chemotherapy regimen necessitates a comprehensive interdisciplinary discussion and a meticulous assessment of the risks and benefits that is customized to each individual patient. Flagellate dermatitis can reoccur with bleomycin re-exposure; a combined approach of proactive topical and systemic steroid treatment seems to diminish the likelihood of FD recurrence.5
Our case underscores the importance of recognizing, detecting, and managing FD promptly in individuals undergoing bleomycin-based chemotherapy. Medical professionals should familiarize themselves with this distinct adverse effect linked to bleomycin, enabling prompt discontinuation if necessary, and educate patients about the condition’s typically temporary nature, thereby alleviating their concerns.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol. 2014;80:149-152.
- Ziemer M, Goetze S, Juhasz K, et al. Flagellate dermatitis as a bleomycinspecific adverse effect of cytostatic therapy: a clinical-histopathologic correlation. Am J Clin Dermatol. 2011;12:68-76. doi:10.2165/11537080-000000000-00000
- Moulin G, Fière B, Beyvin A. Cutaneous pigmentation caused by bleomycin. Article in French. Bull Soc Fr Dermatol Syphiligr. 1970;77:293-296.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503. doi:10.4103/0973-1482.119358
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi:10.1136/bmj.m4087
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol. 2014;80:149-152.
- Ziemer M, Goetze S, Juhasz K, et al. Flagellate dermatitis as a bleomycinspecific adverse effect of cytostatic therapy: a clinical-histopathologic correlation. Am J Clin Dermatol. 2011;12:68-76. doi:10.2165/11537080-000000000-00000
- Moulin G, Fière B, Beyvin A. Cutaneous pigmentation caused by bleomycin. Article in French. Bull Soc Fr Dermatol Syphiligr. 1970;77:293-296.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503. doi:10.4103/0973-1482.119358
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi:10.1136/bmj.m4087
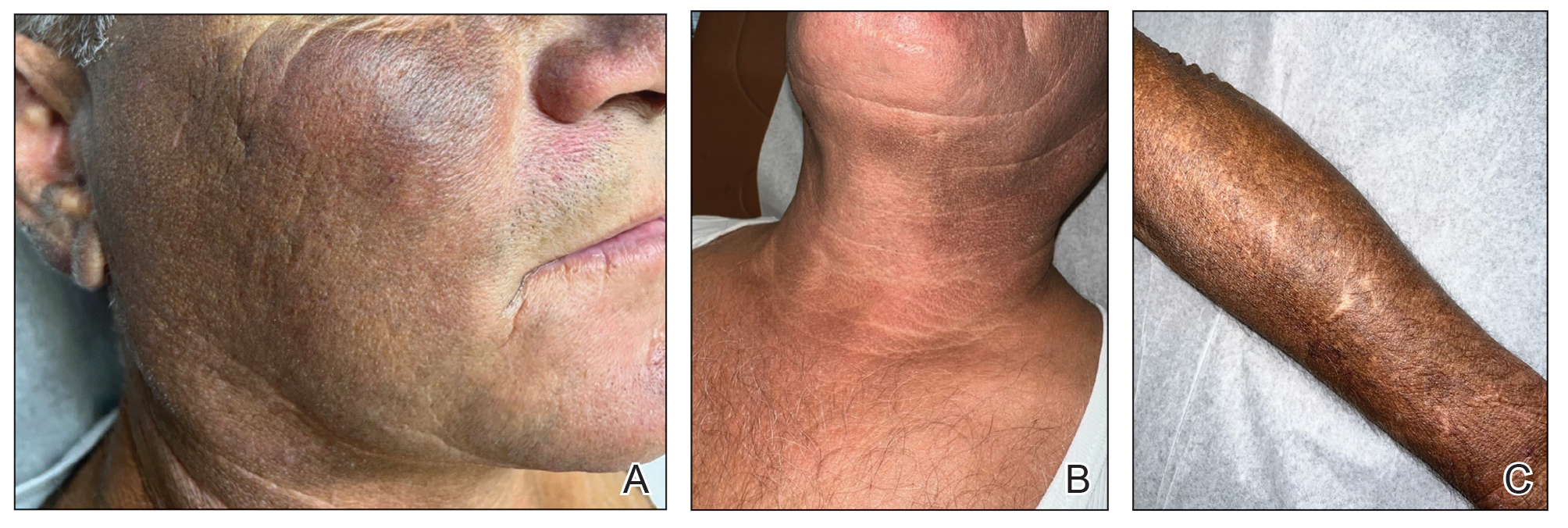
A 28-year-old man presented for evaluation of an intensely itchy rash of 5 days’ duration involving the face, trunk, arms, and legs. The patient recently had been diagnosed with classical Hodgkin lymphoma and was started on a biweekly chemotherapy regimen of adriamycin, bleomycin, vinblastine, and dacarbazine 3 weeks prior. He reported that a red, itchy, papular rash had developed on the hands 1 week after starting chemotherapy and improved with antihistamines. Symptoms of the current rash included night sweats, occasional fever, substantial unintentional weight loss, and fatigue. He had no history of urticaria, angioedema, anaphylaxis, or nail changes.
Physical examination revealed widespread, itchy, linear and curvilinear hyperpigmented plaques on the upper arms, shoulders, back (top), face, and thighs, as well as erythematous grouped papules on the bilateral palms (bottom). There was no mucosal or systemic involvement.
Rare Case of Photodistributed Hyperpigmentation Linked to Kratom Consumption
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
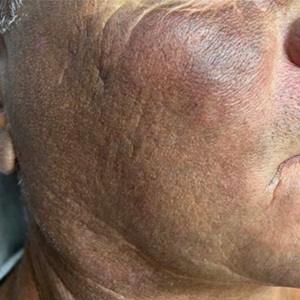
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.
When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
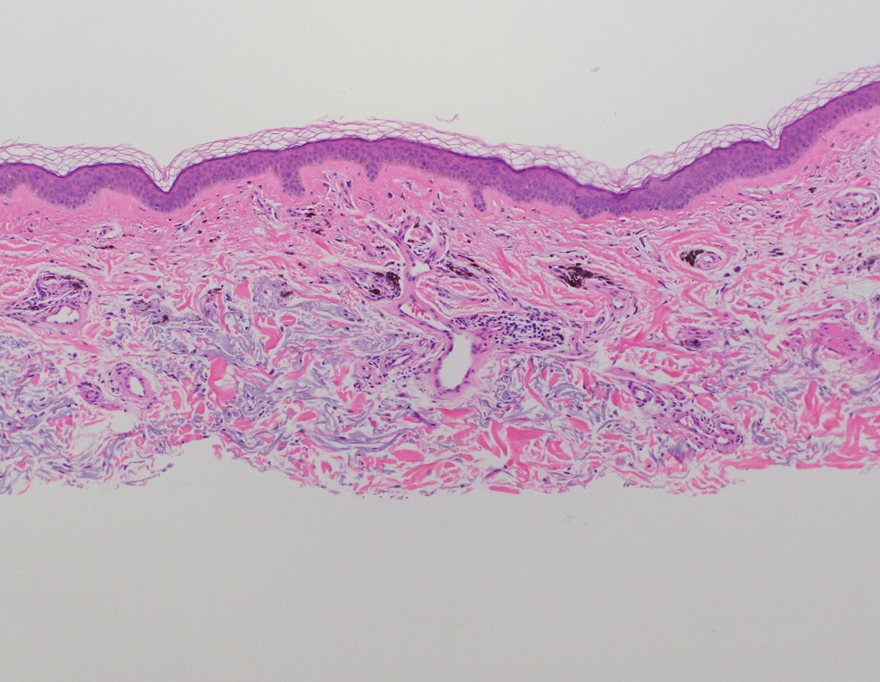
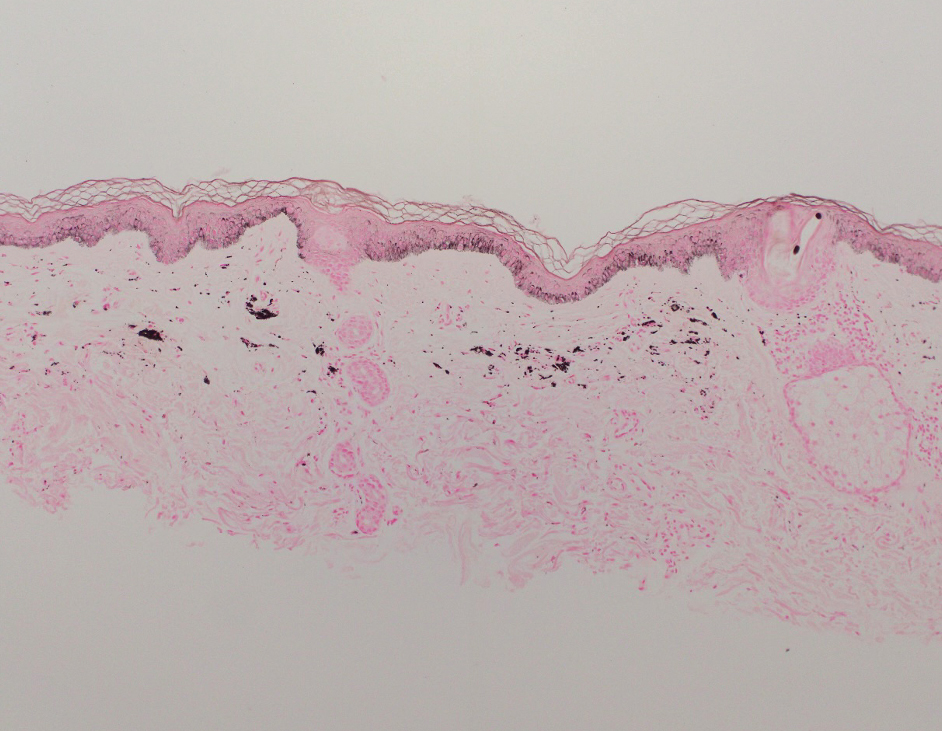
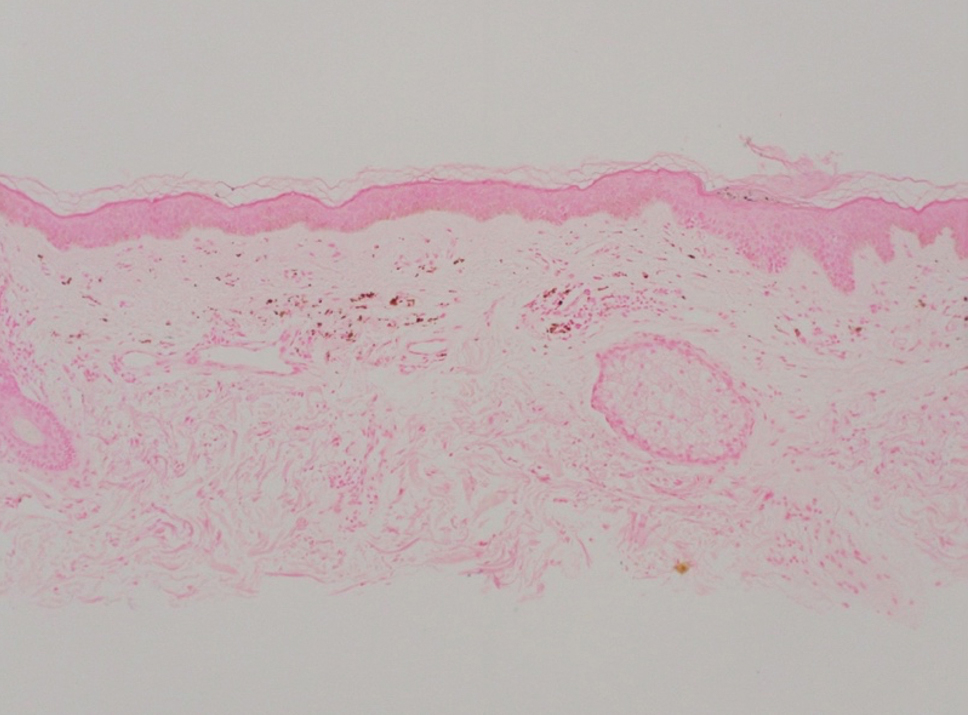
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).
Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
Practice Points
- Clinicians should be aware of photodistributed hyperpigmentation as a potential adverse effect of kratom usage.
- Kratom-induced photodistributed hyperpigmentation should be suspected in patients with hyperpigmented lesions in sun-exposed areas of the skin following kratom use. A biopsy of lesions should be obtained to confirm the diagnosis.
- Cessation of kratom should be recommended.
Unlocking the Potential of Baricitinib for Vitiligo
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
Enhanced Care for Pediatric Patients With Generalized Lichen Planus: Diagnosis and Treatment Tips
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.
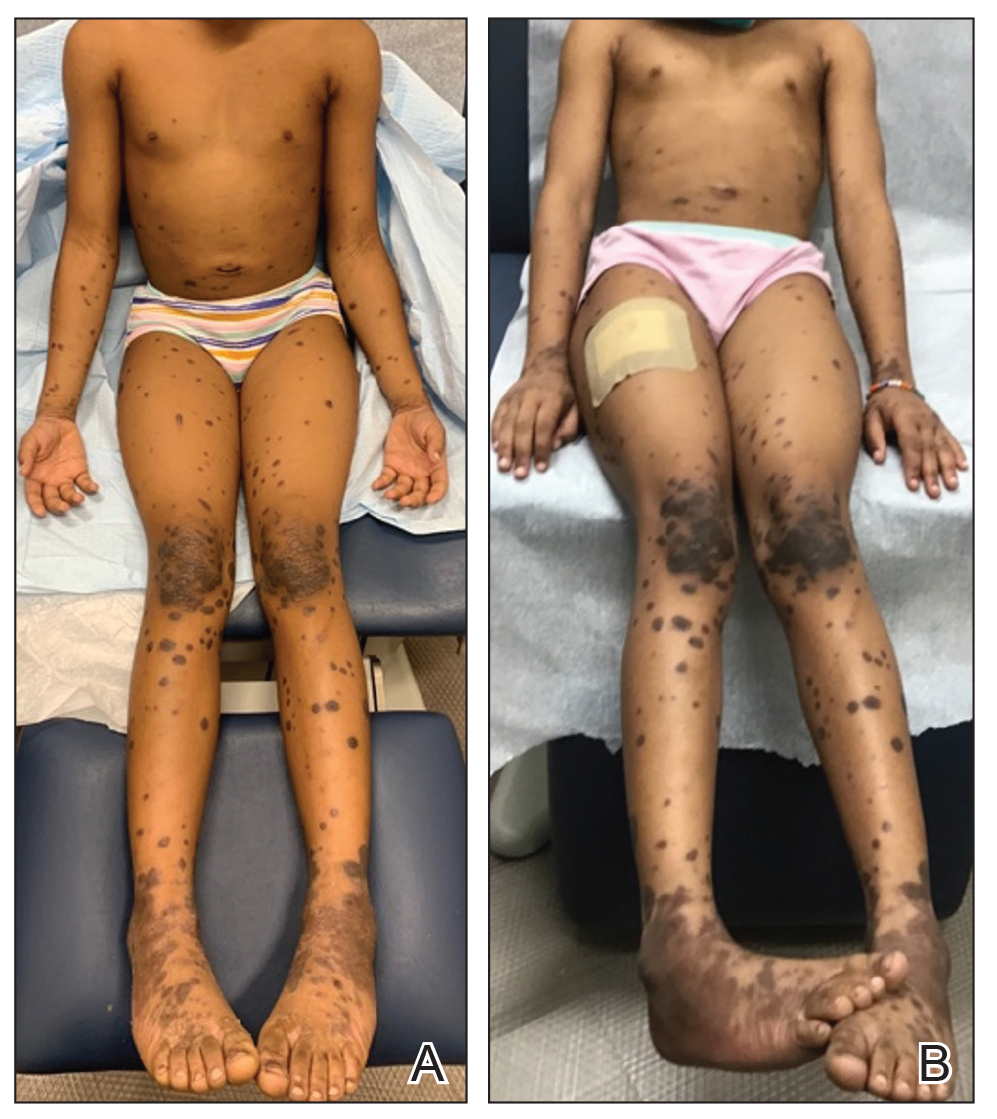
The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Managing Vitiligo: Combination Therapies, New Treatments
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Black Children With Vitiligo at Increased Risk for Psychiatric Disorders: Study
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Storybooks Can Help Children Deal with Skin Conditions
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
The Use of Tranexamic Acid and Microneedling in the Treatment of Melasma: A Systematic Review
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.
Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19
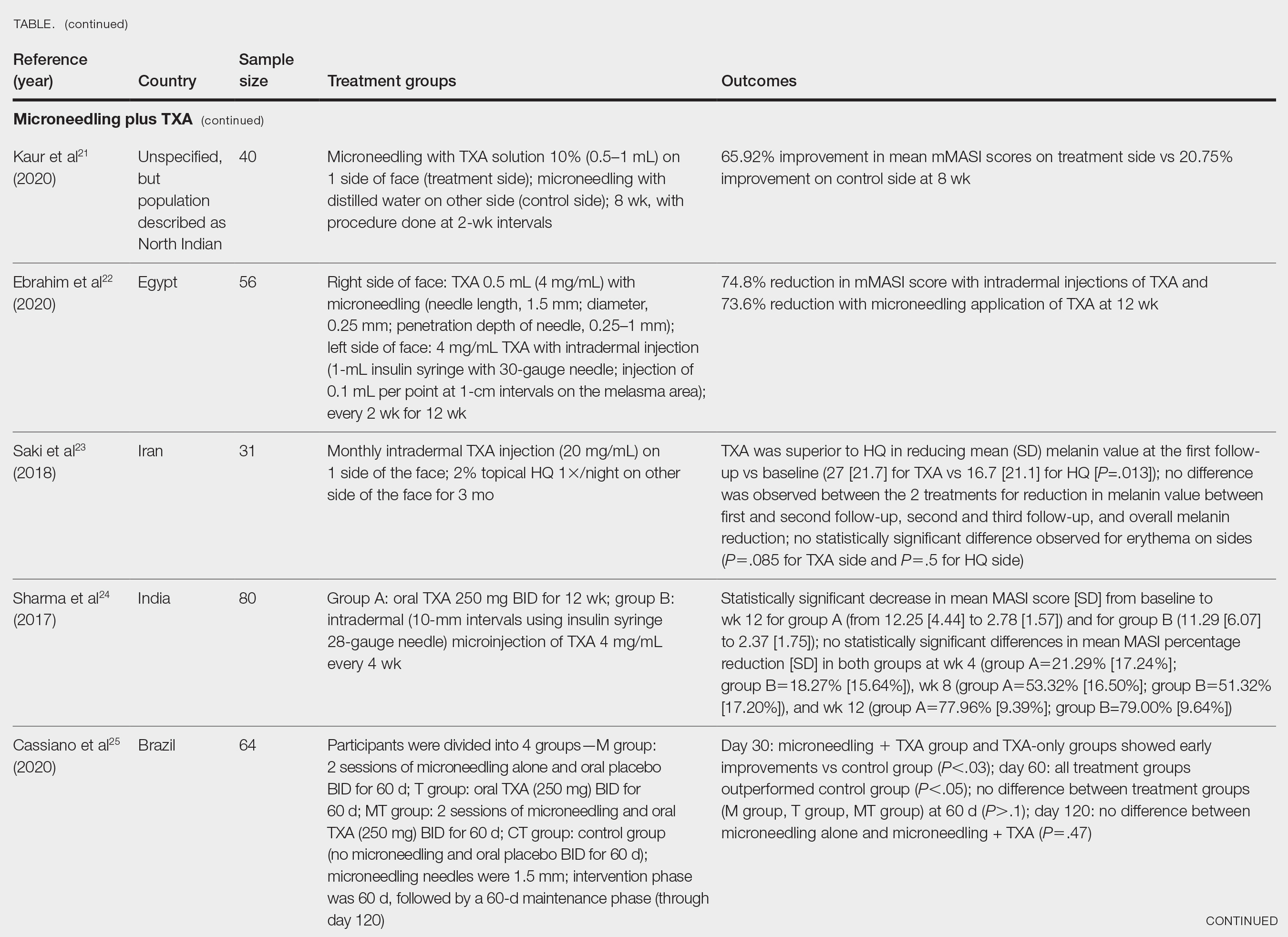
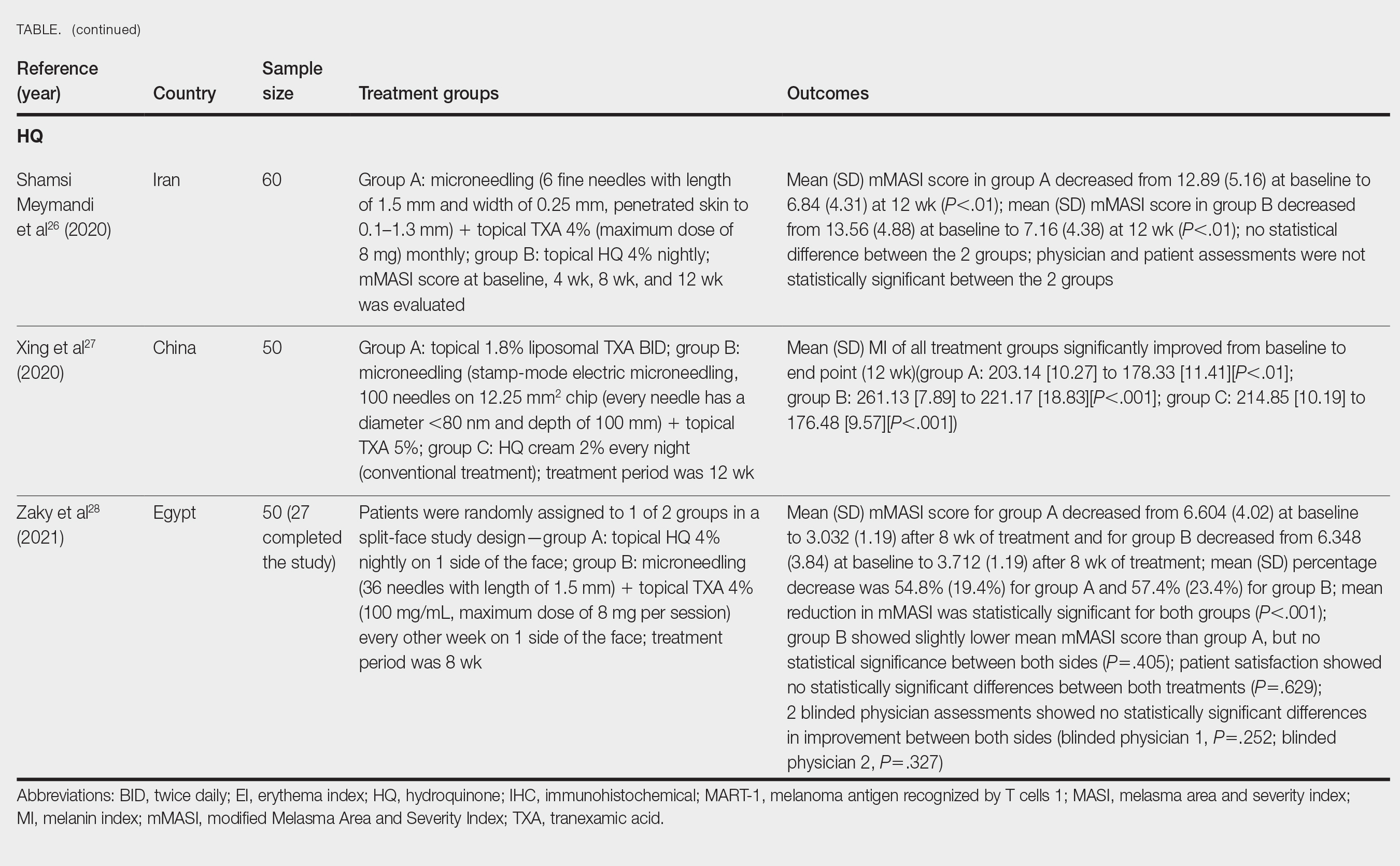
Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.

Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19



Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.

Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19



Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
Practice Points
- Combination therapy with tranexamic acid (TXA) and microneedling is a safe and effective treatment for melasma.
- Combining TXA with microneedling may result in decreased melasma relapse rates.
Could Targeting ‘Zombie Cells’ Extend a Healthy Lifespan?
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.