User login
The State of Skin of Color Centers in the United States: A Cross-Sectional Survey Study
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
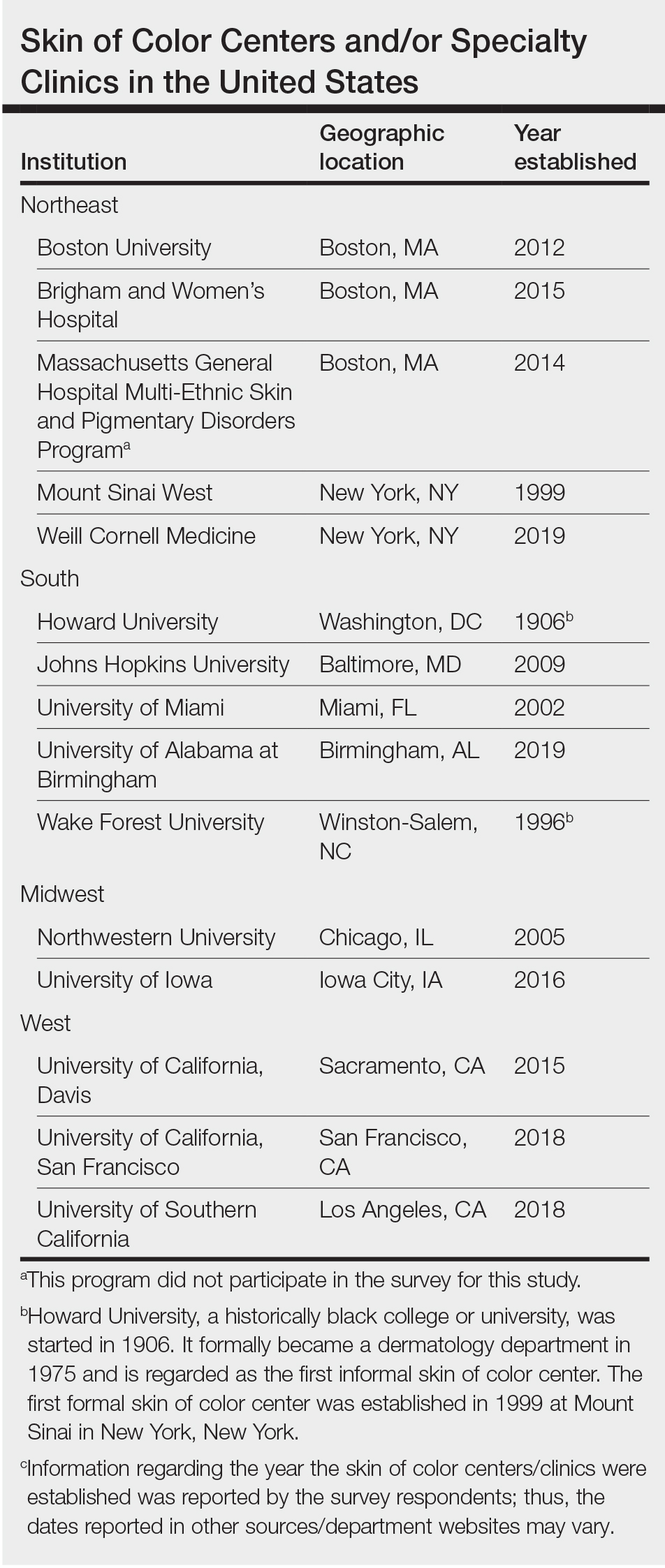
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.
Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).
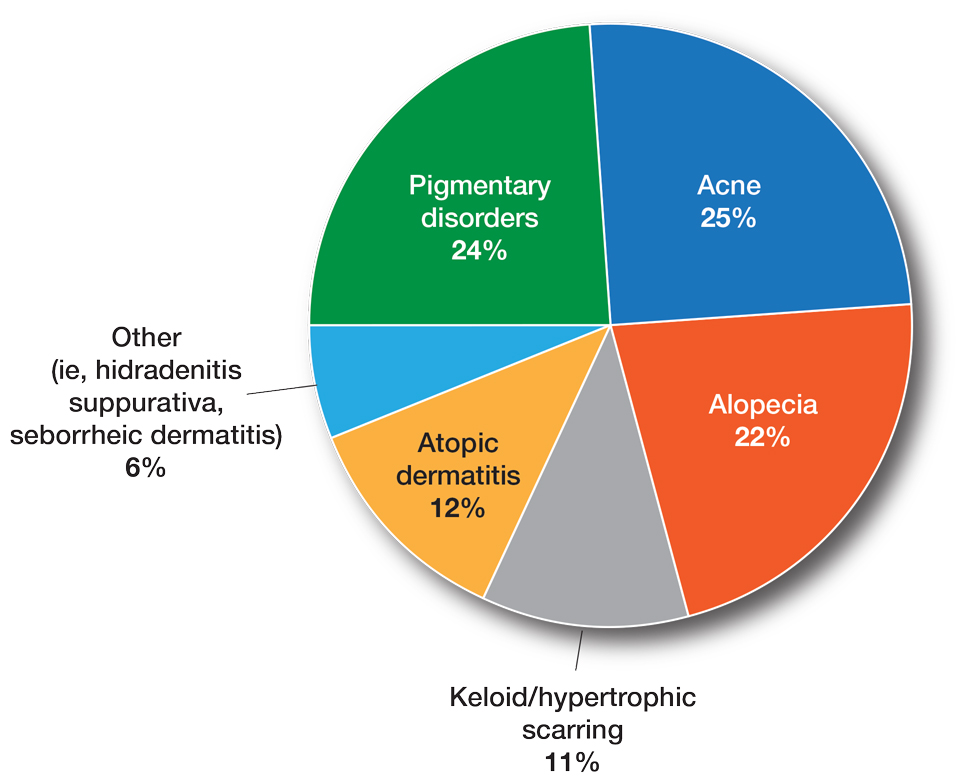
The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
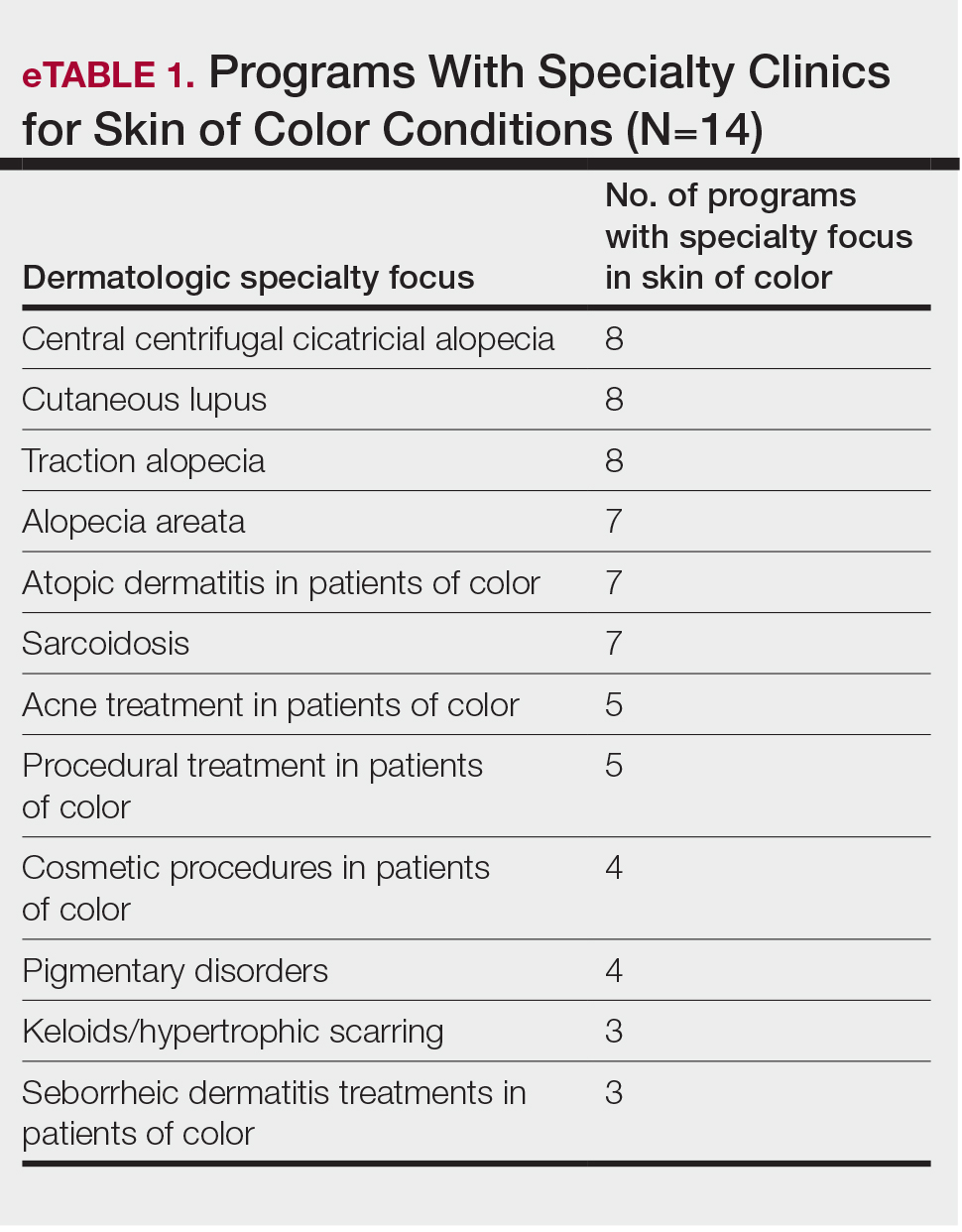
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix

- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Practice Points
- Skin of color centers in the United States work to reverse the paucity of research, education, and training in skin of color dermatology and promote the diversification of residents and faculty.
- Skin of color centers expand access to culturally competent and inclusive care for diverse patient populations.
Advancements in Targeted Therapies for Vitiligo: Prioritizing Equity in Drug Development
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
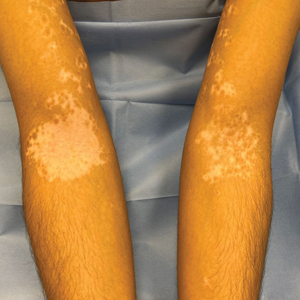
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).
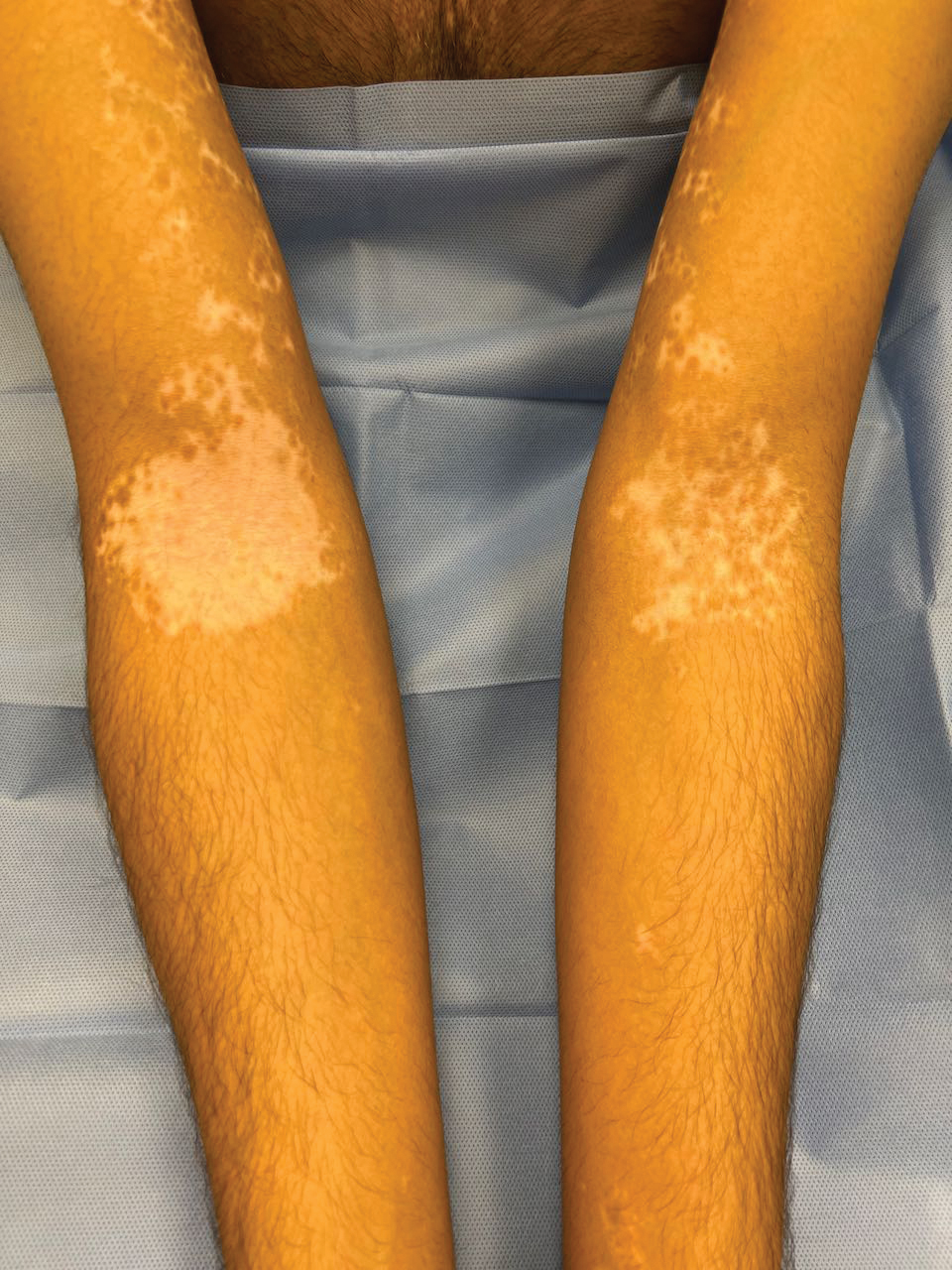
Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
Practice Points
- Vitiligo is an autoimmune disease of the skin that affects all skin types but can be particularly disfiguring in those with skin of color.
- Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is the only US Food and Drug Administration–approved treatment to repigment the skin in vitiligo and has shown efficacy for individuals with all skin phototypes.
- Individuals with skin of color are underrepresented in patient cohorts for JAK inhibitor clinical trials for vitiligo, mirroring a phenomenon seen in the majority of clinical trials. Ensuring diverse participant enrollment and measuring quality-of-life metrics will strengthen future clinical trials for treatment of vitiligo and other skin diseases impacting patients with skin of color.
Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28
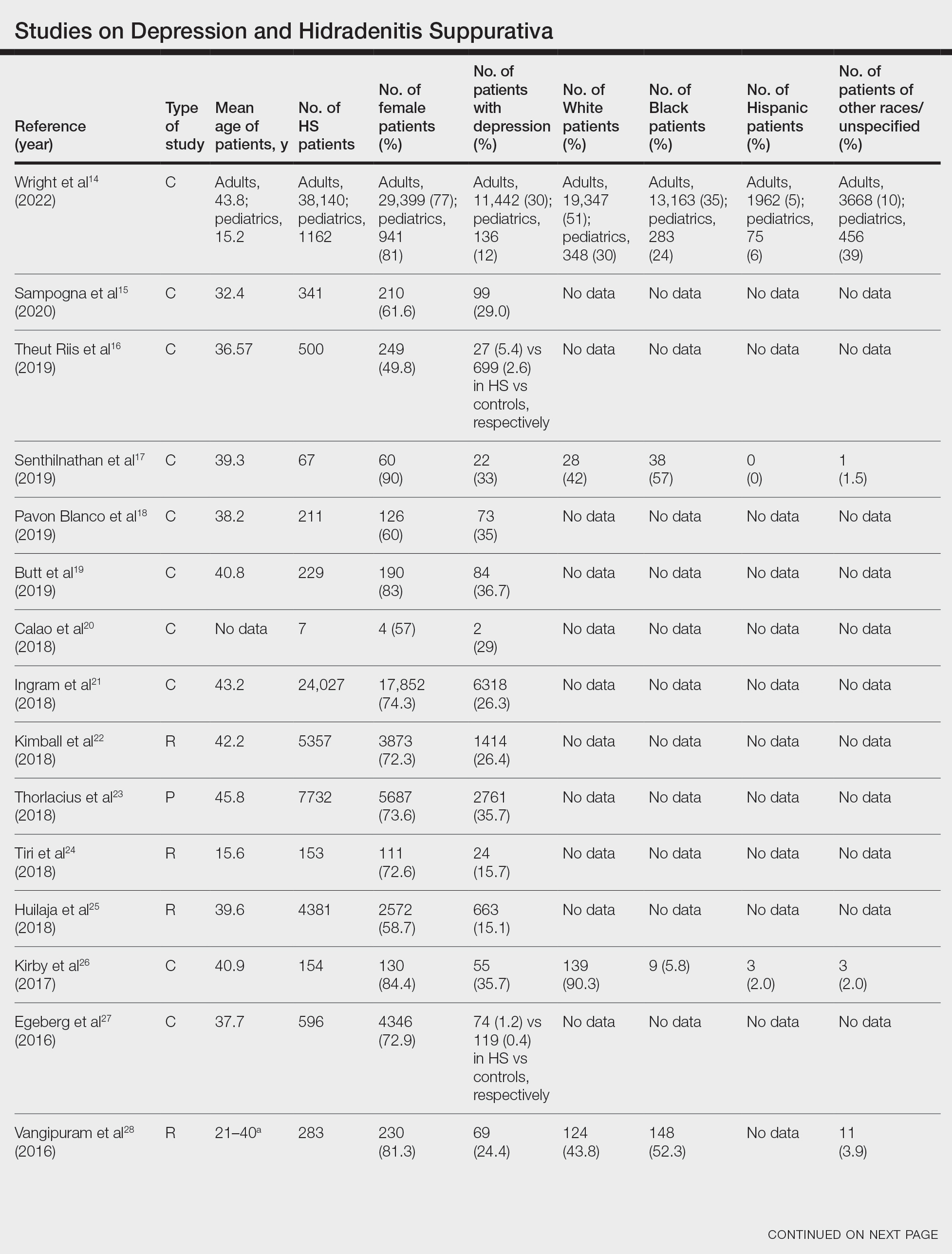
Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Practice Points
- Hidradenitis suppurativa (HS) is known to be associated with systemic inflammation and comorbidities, including depression.
- Depression may be a potential contributing factor to HS in affected patients, and studies on HS with comorbid depression in patients with skin of color are lacking.
Analysis of Online Diet Recommendations for Vitiligo
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.
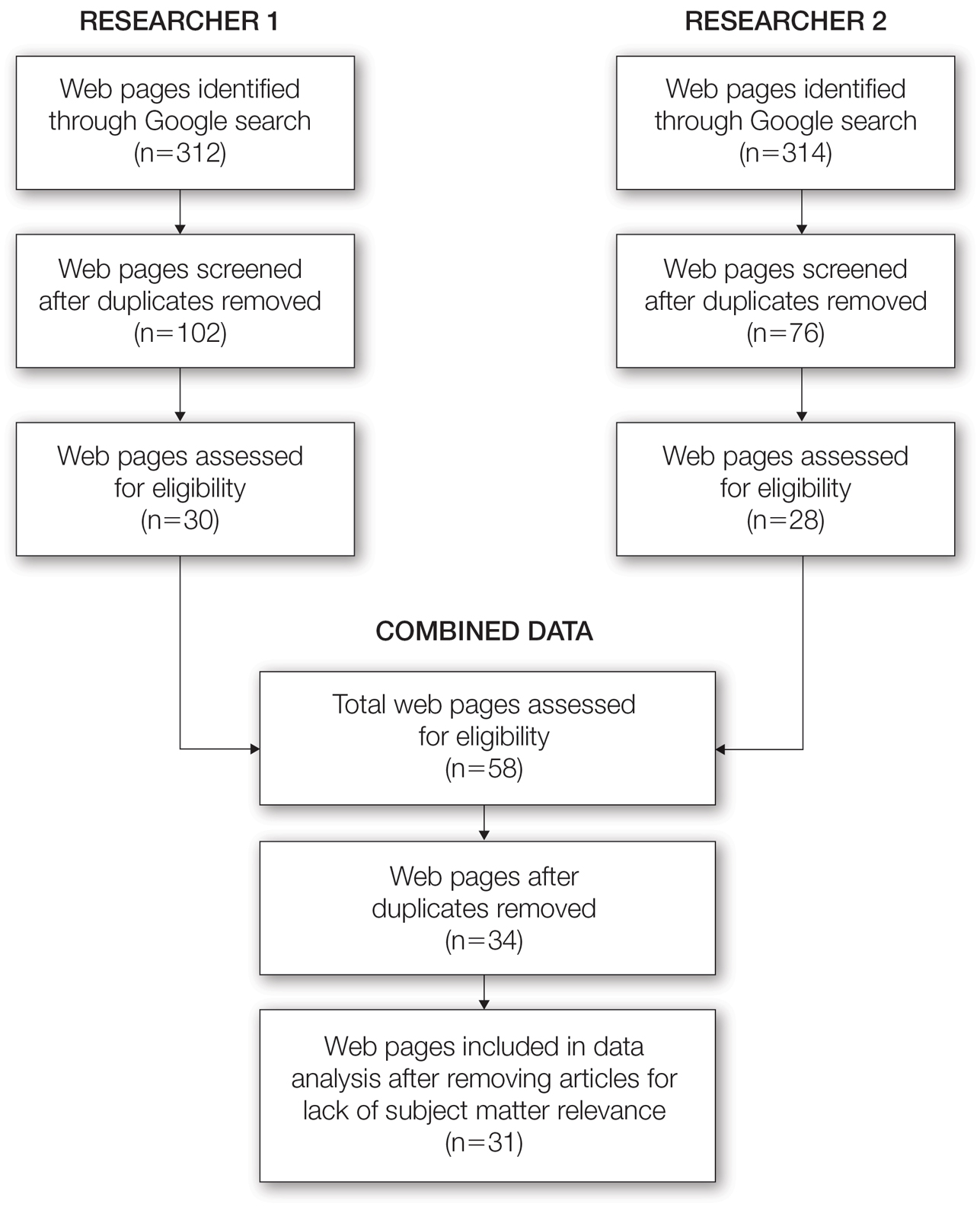
From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).
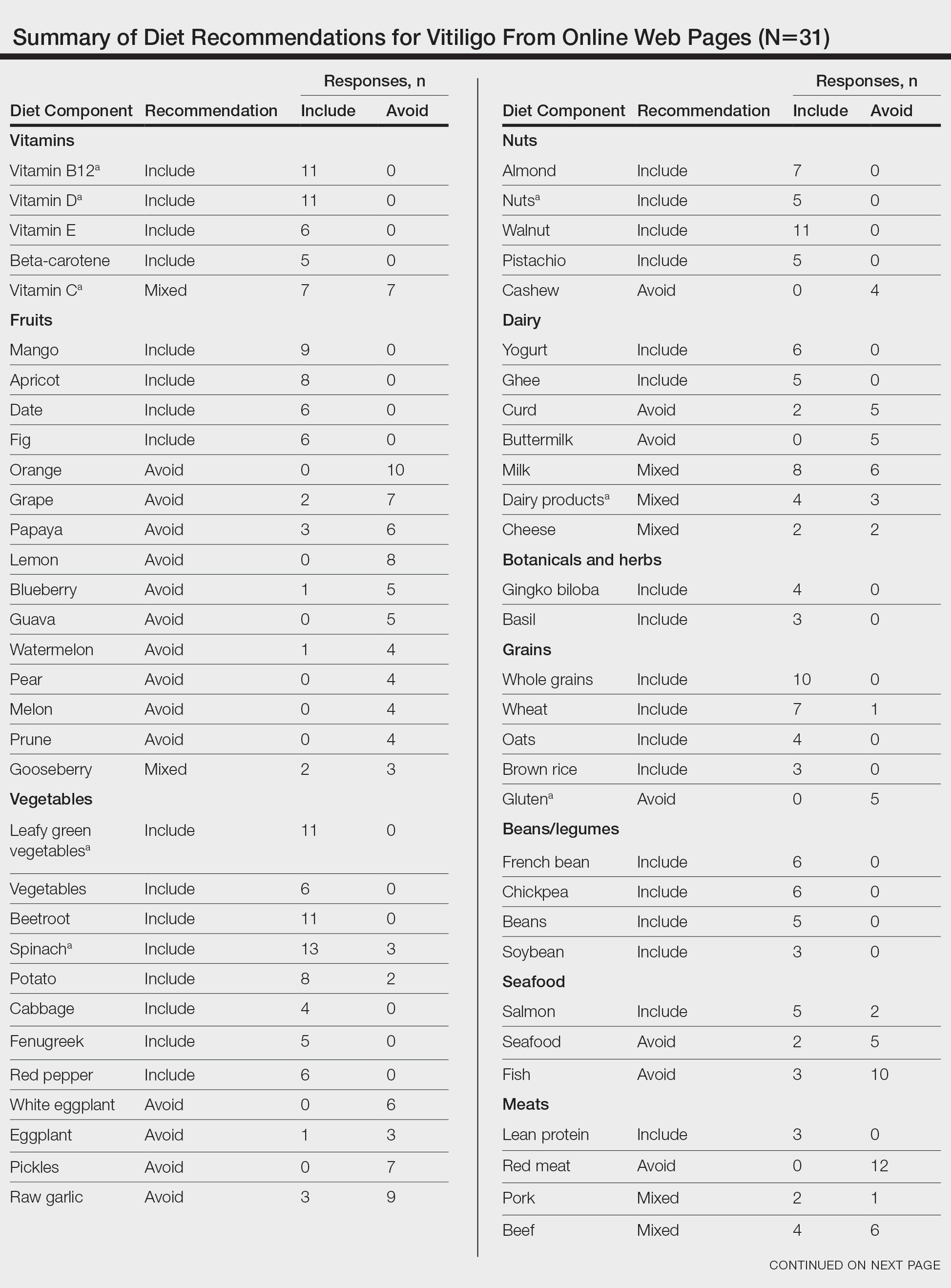
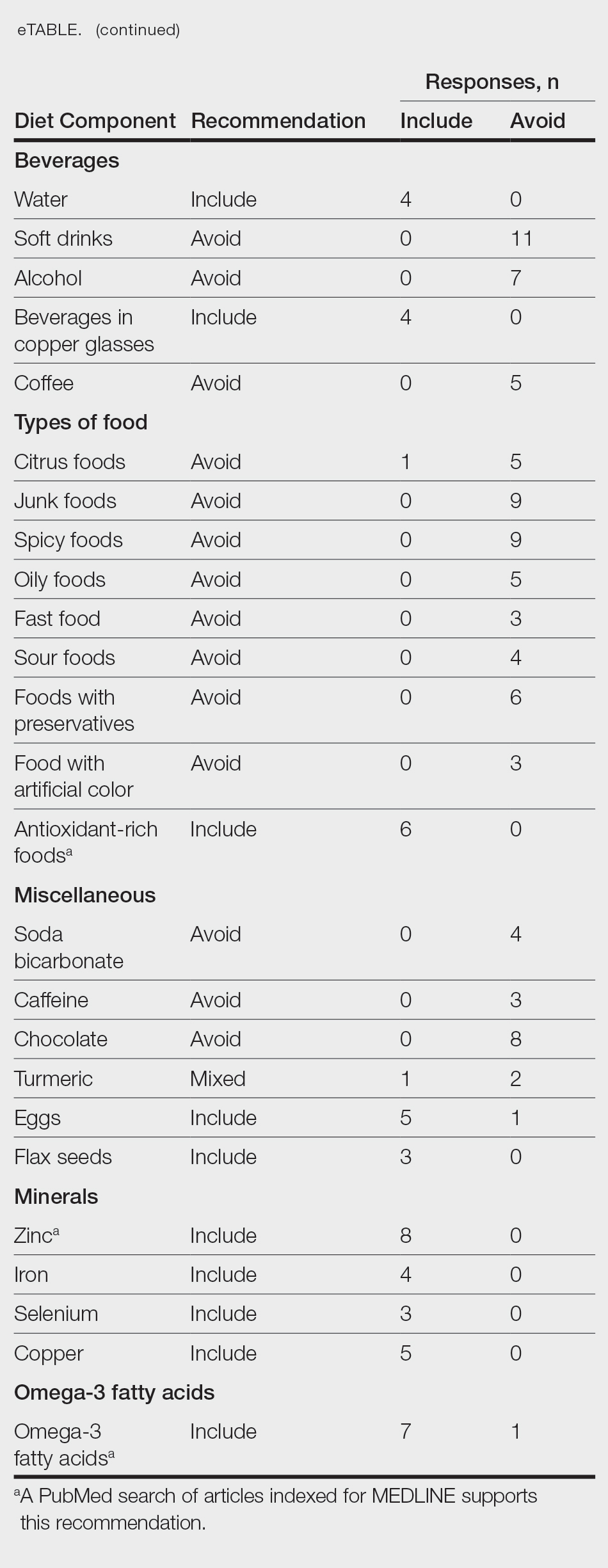
For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Practice Points
- There are numerous online dietary and supplement recommendations that claim to impact vitiligo but most are not authored by medical professionals or dermatologists.
- Scientific evidence supporting specific dietary and supplement recommendations for vitiligo is limited.
- Current preliminary data support the potential recommendation for dietary supplementation with vitamin D, vitamin B12, zinc, and omega-3 fatty acids.
Attitudes Toward Utilization of Minimally Invasive Cosmetic Procedures in Black Women: Results of a Cross-sectional Survey
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.
Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.
Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).
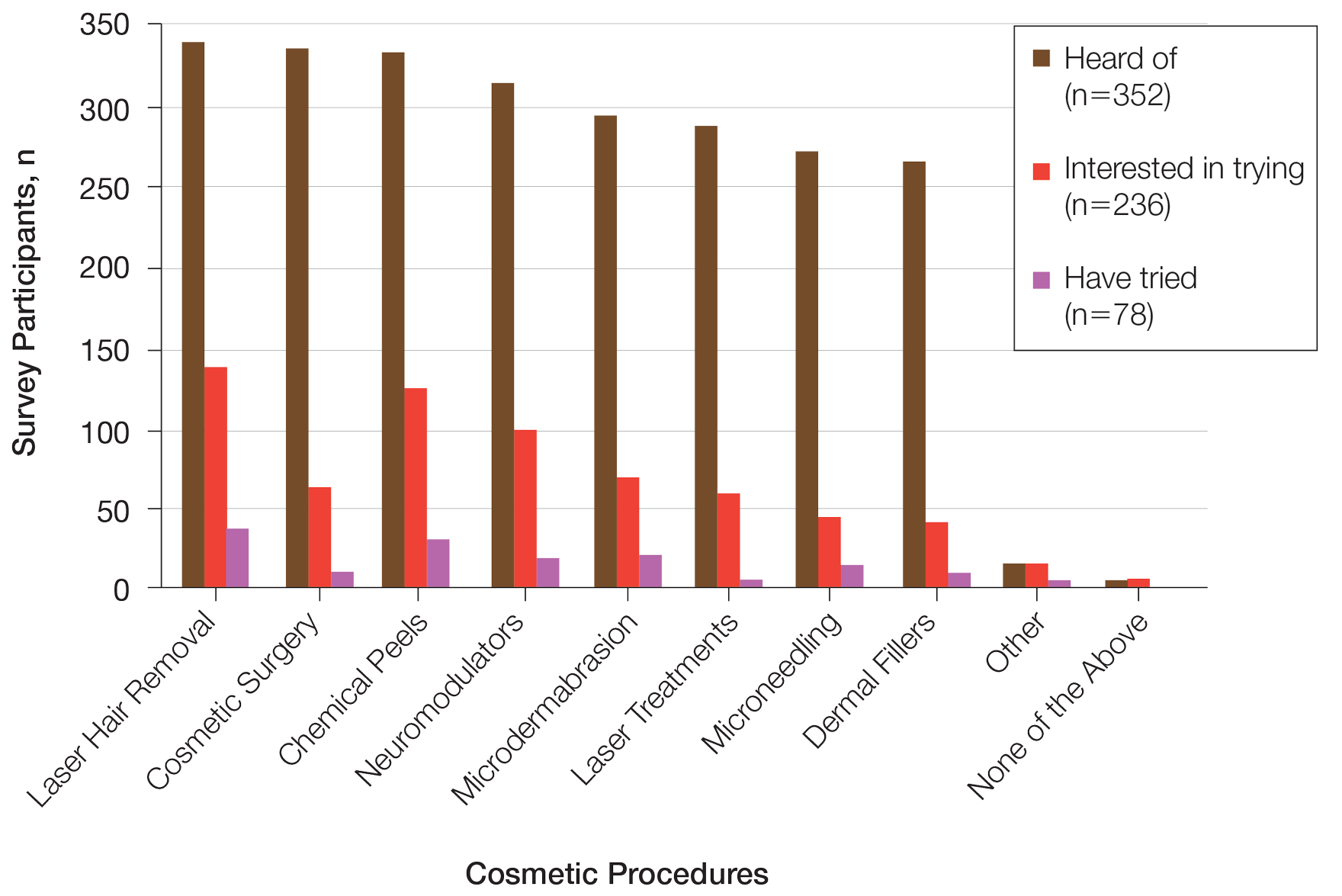
Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Practice Points
- Cosmetic procedures may be more widely accepted among younger Black women than older Black women.
- Age has a considerable influence on the types of cosmetic procedures that Black women are interested in trying.
- Microdermabrasion, chemical peels, and laser hair removal were the most frequently utilized procedures in this study population.
- As attitudes and perceptions of young Black women are changing and favoring more frequent sunscreen use, dermatologists should remain on top of current trends to provide culturally sensitive and relevant recommendations to patients with darker skin tones.
Improving Diagnostic Accuracy in Skin of Color Using an Educational Module
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.
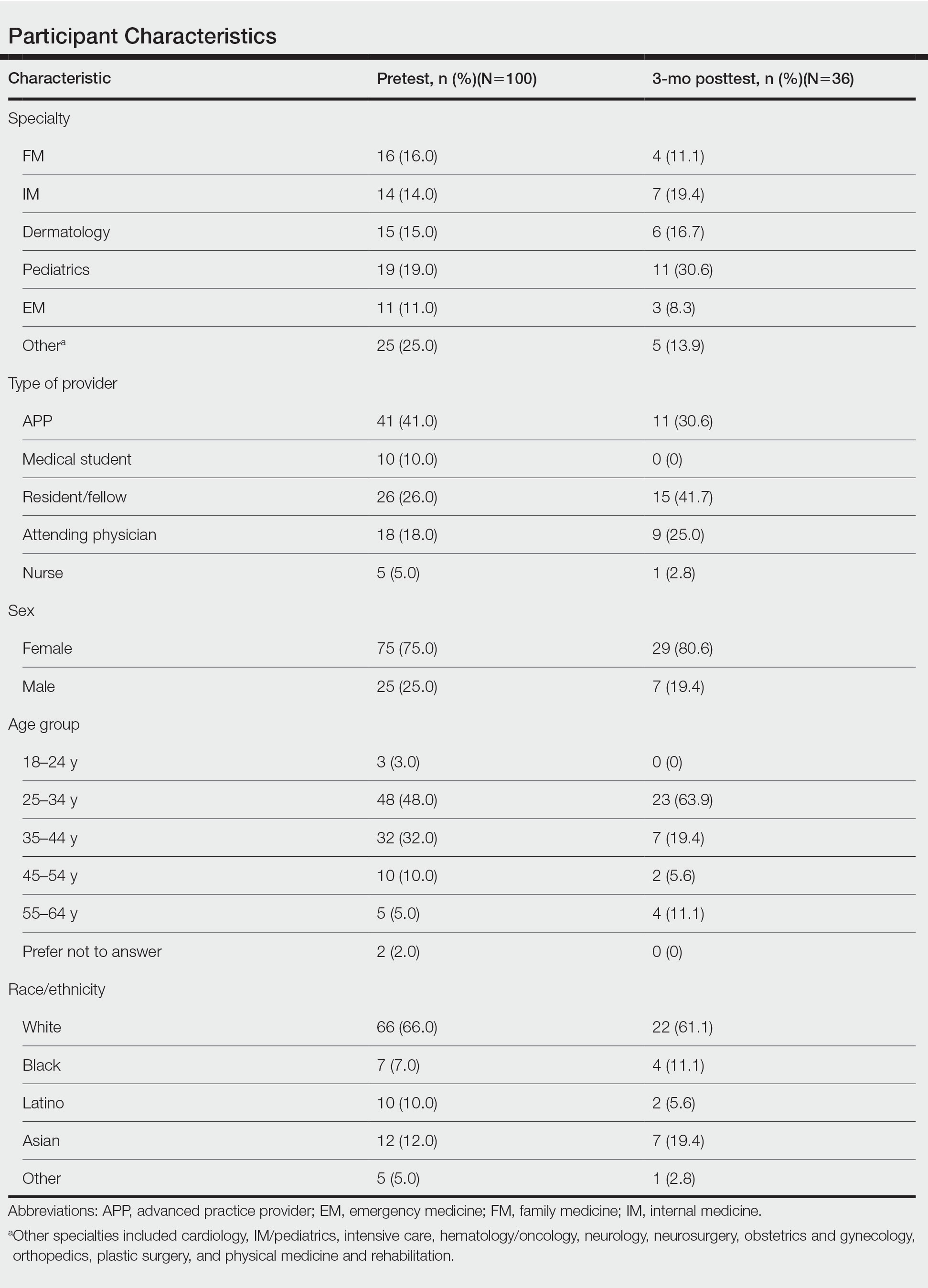
Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).
Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.
Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.

Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).

Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.

Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.

Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).

Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.

Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
Practice Points
- Disparities exist among interprofessional health care providers when diagnosing conditions in patients with lighter and darker skin, specifically for infectious, cancerous, or inflammatory conditions vs conditions that are preferentially seen in patients with skin of color (SOC).
- A focused educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
Treatment of Frontal Fibrosing Alopecia in Black Patients: A Systematic Review
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.
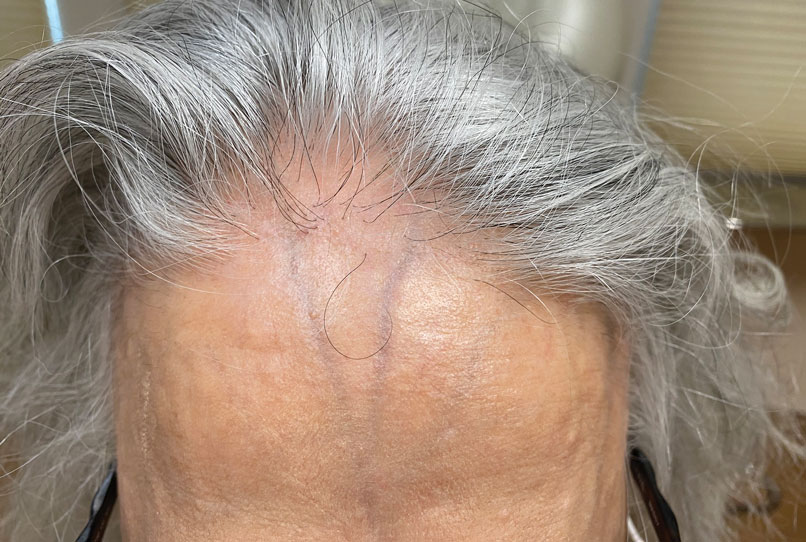
The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.
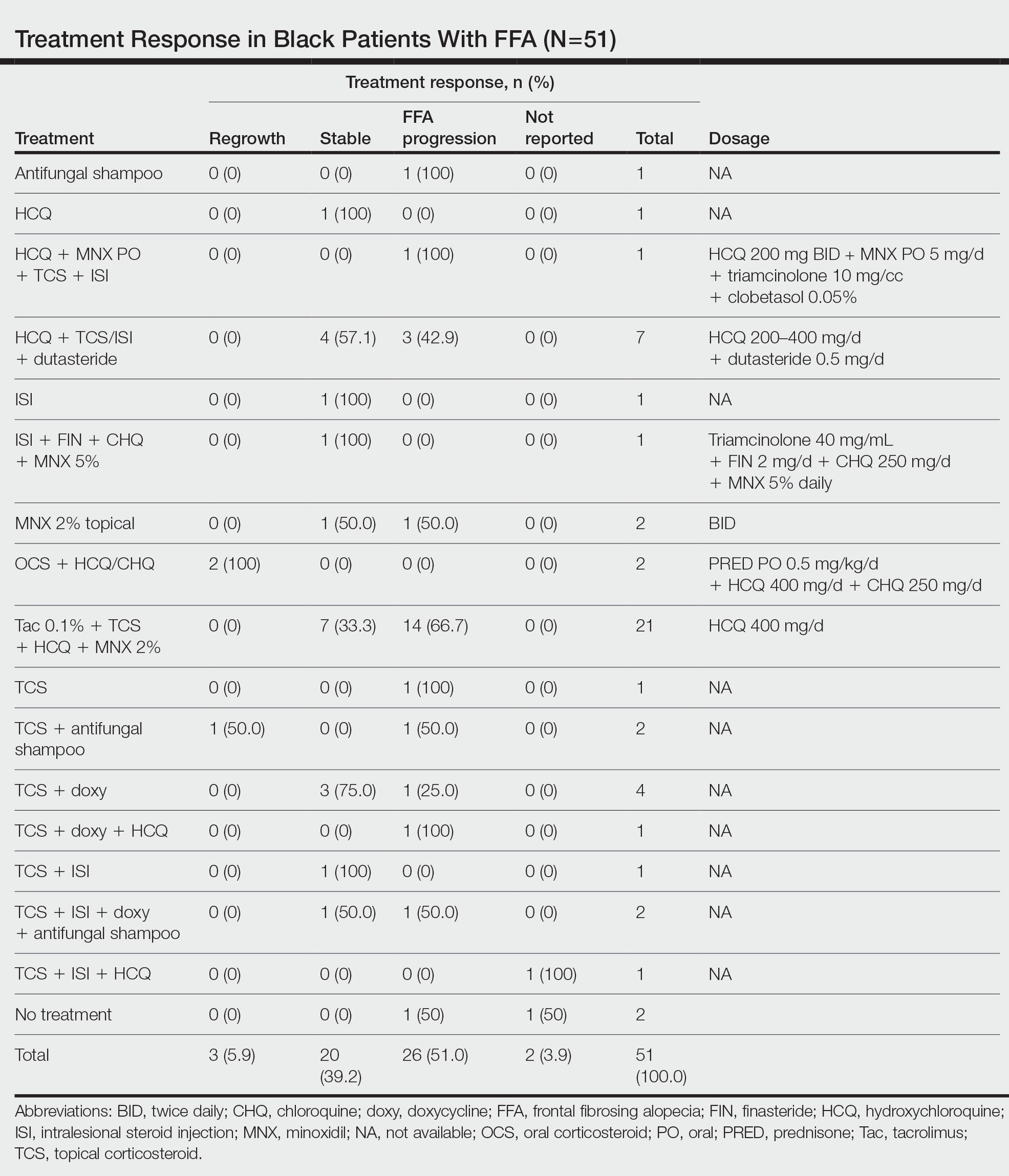
Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.

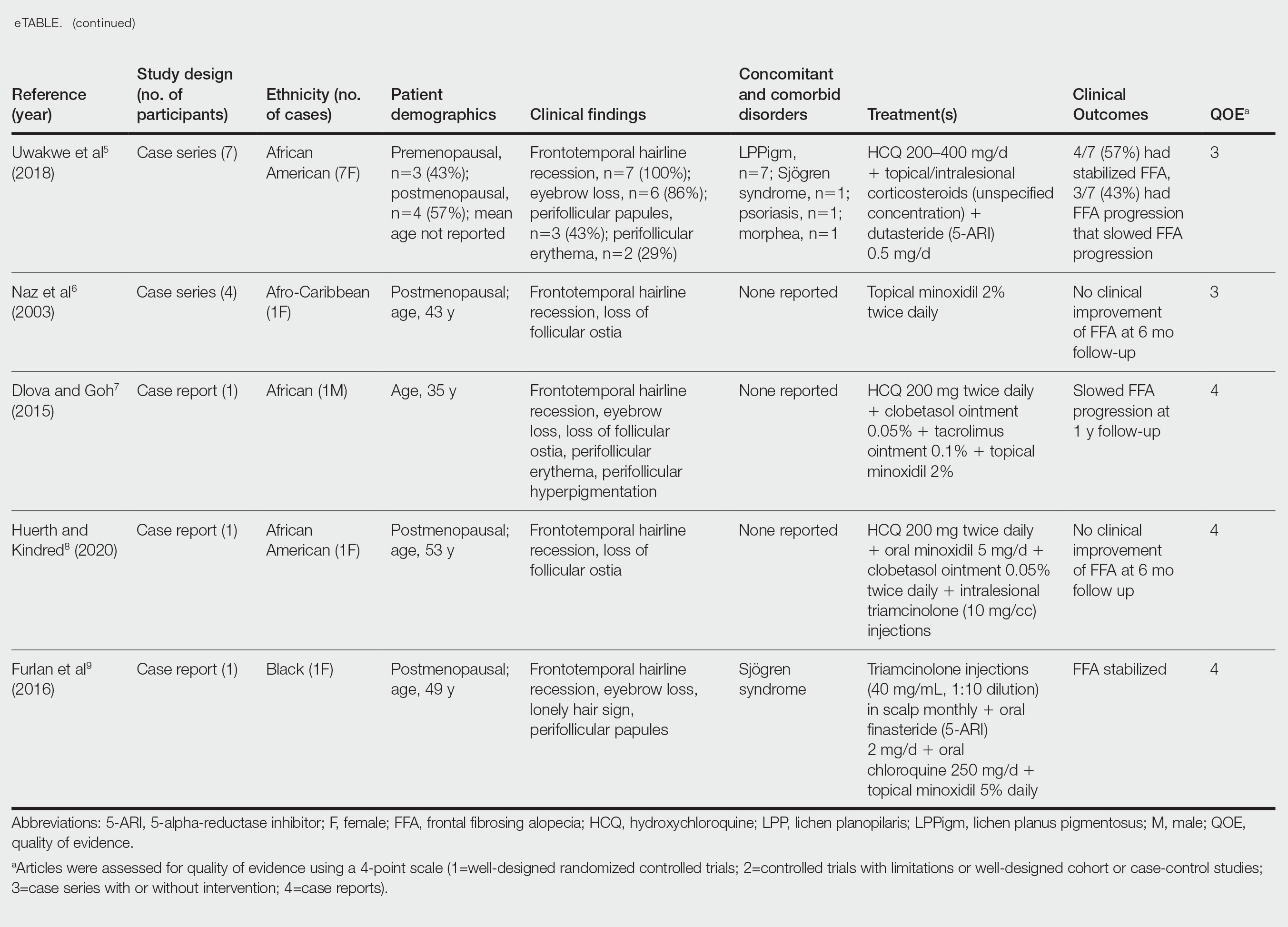
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Practice Points
- Treatment of frontal fibrosing alopecia (FFA) is challenging, and there are no evidence-based treatment guidelines available. Patients with skin of color (SOC) may have varying responses to treatment modalities.
- Special consideration should be taken when treating FFA in patients with SOC.
- Histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
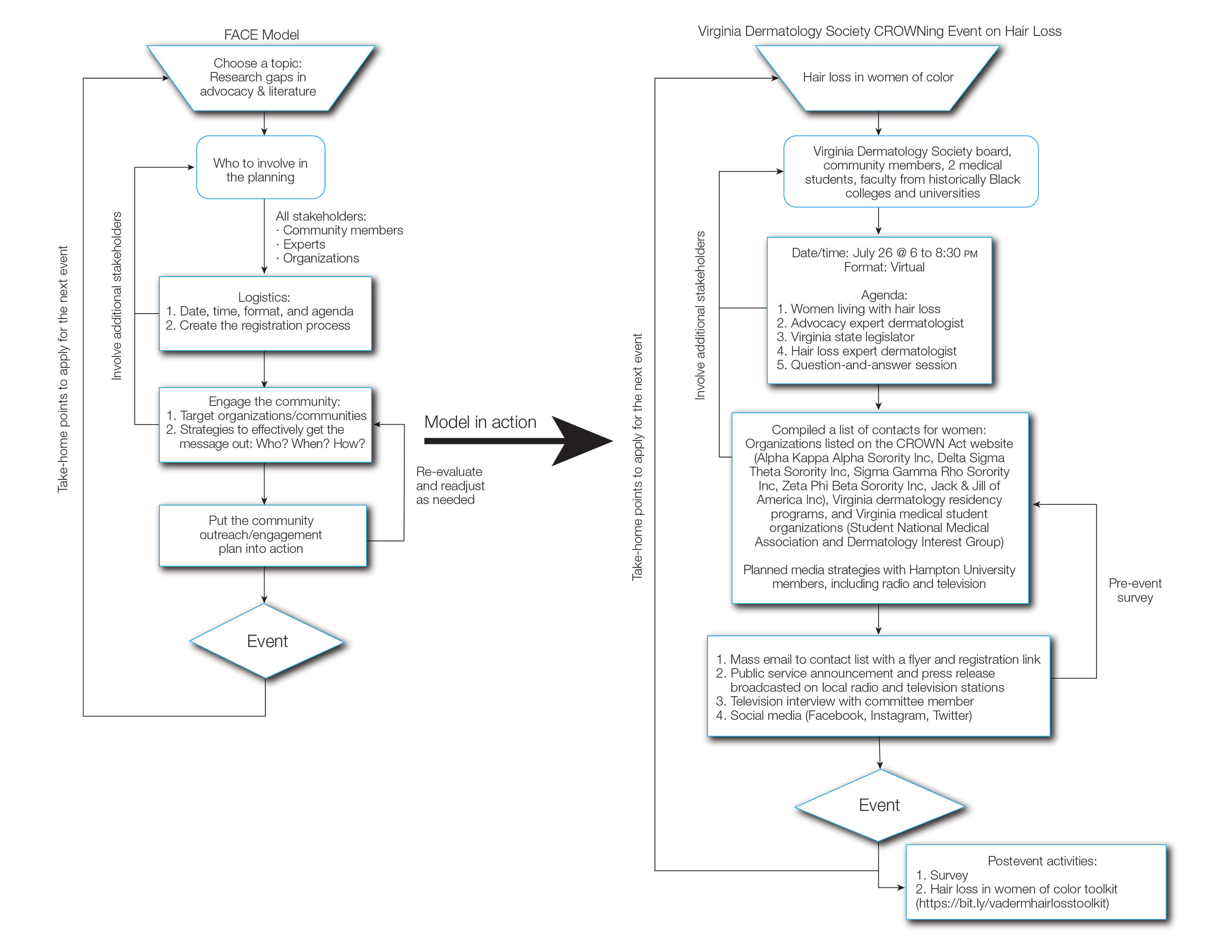
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
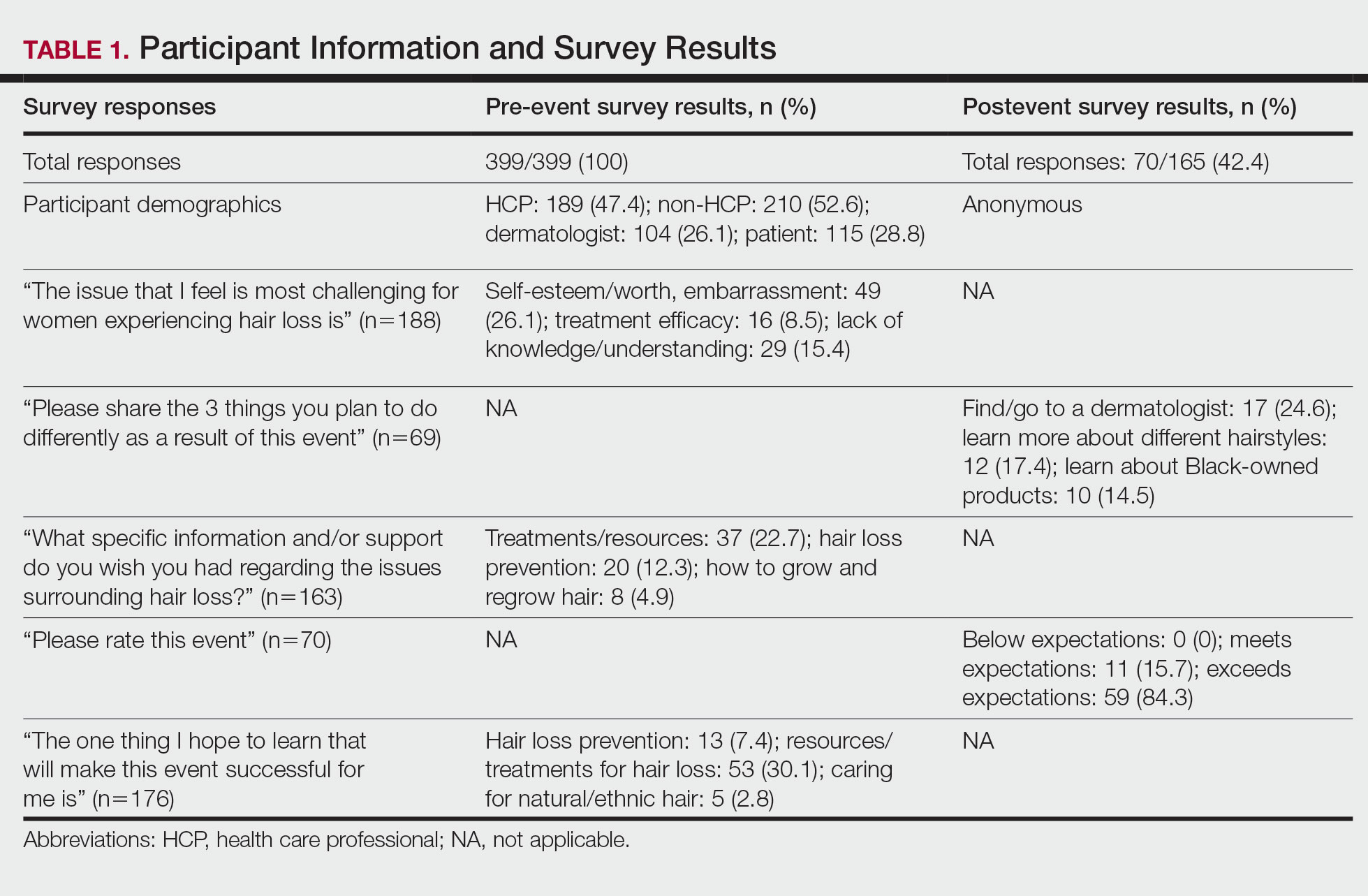
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
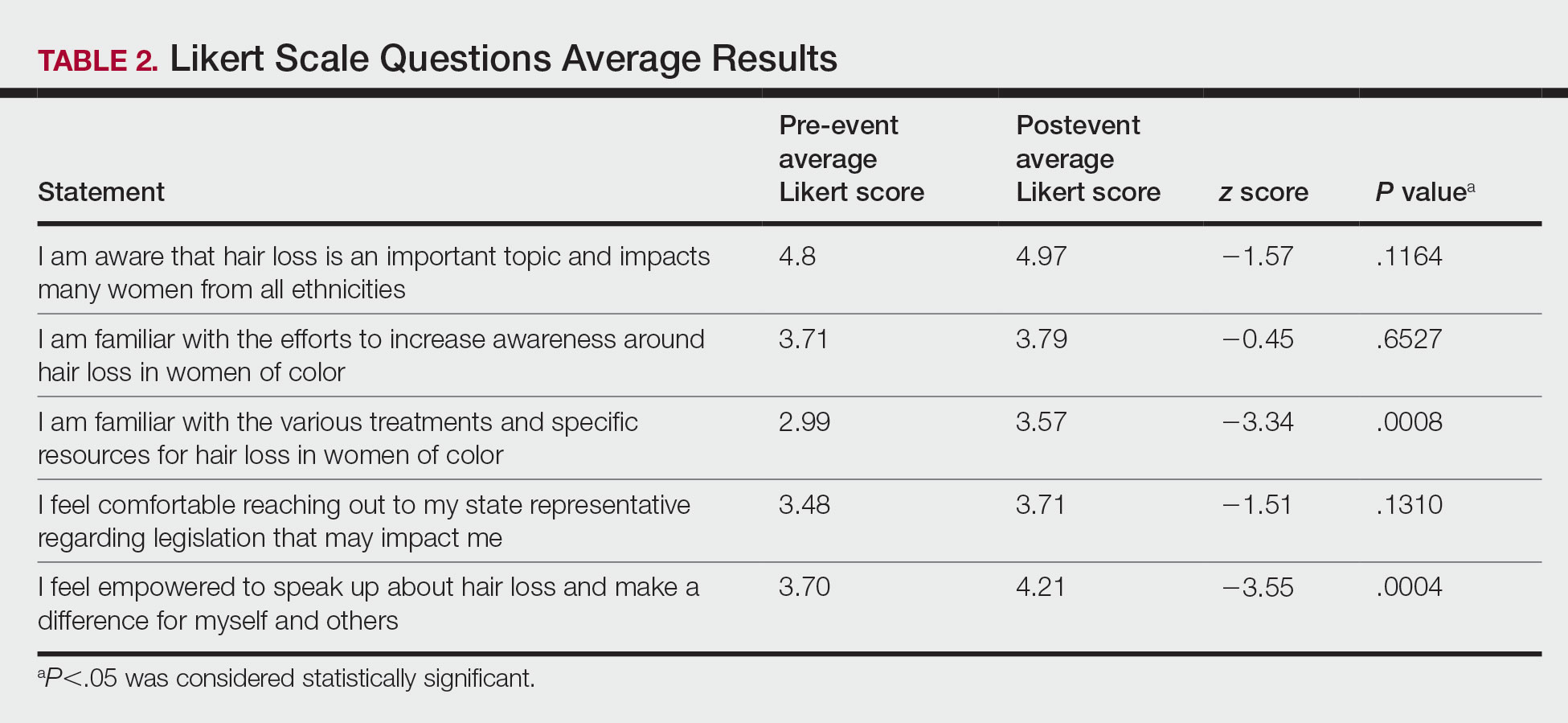
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.
Perceptions of Community Service in Dermatology Residency Training Programs: A Survey-Based Study of Program Directors, Residents, and Recent Dermatology Residency Graduates
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.
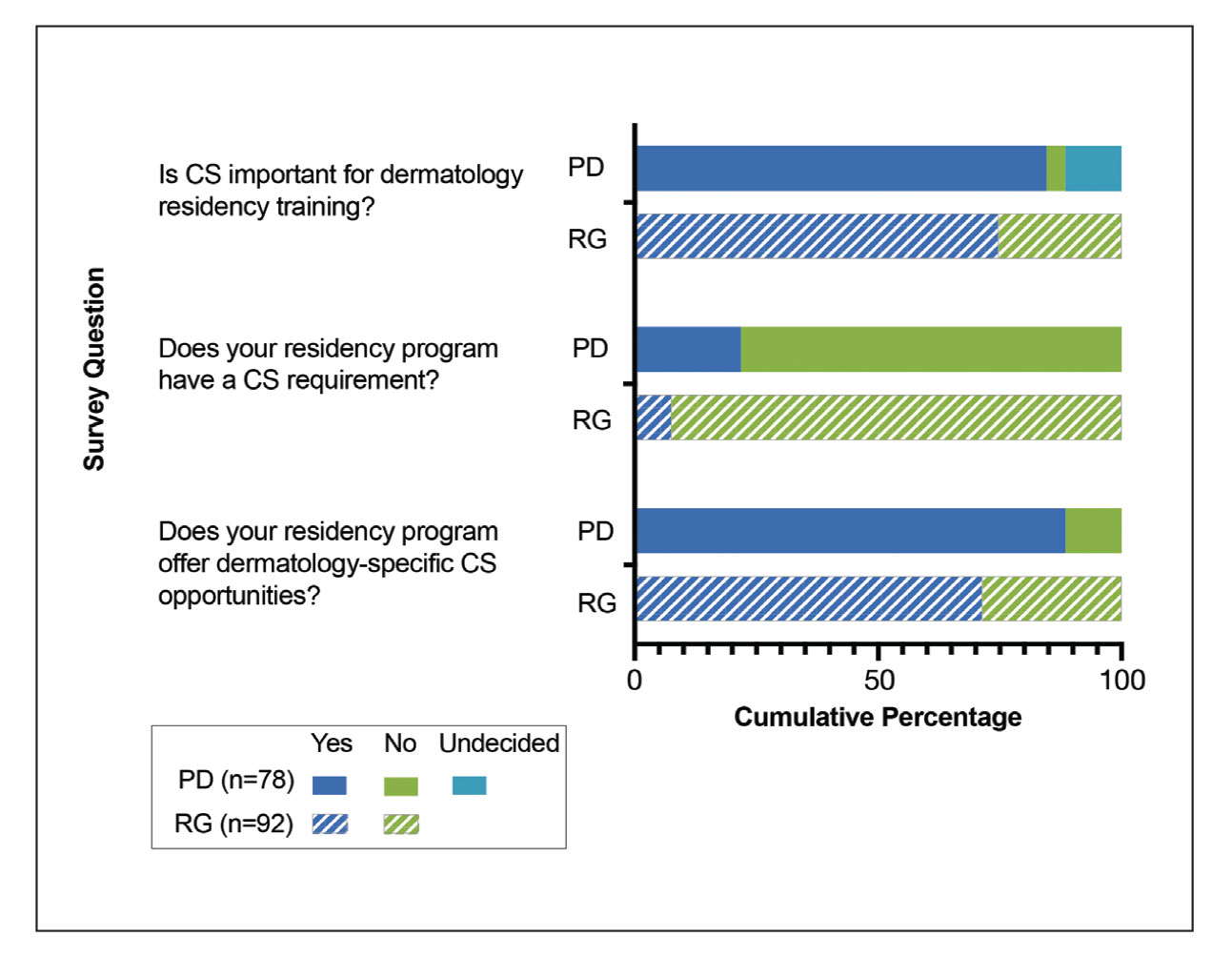
Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).
Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.
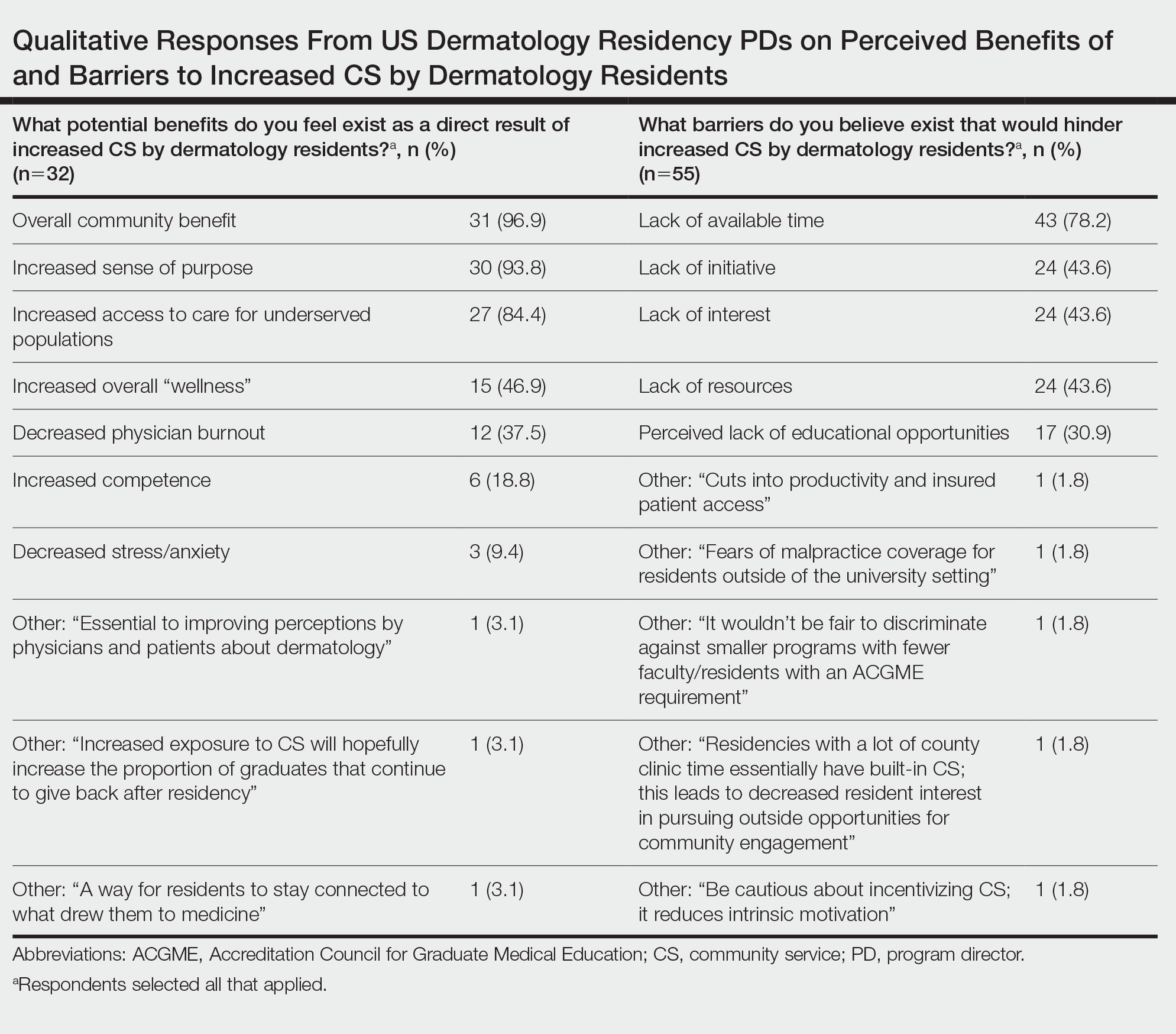
Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Participation of dermatology residents in service-learning experiences increases awareness of health disparities and social factors impacting dermatologic care and promotes a lifelong commitment to serving vulnerable populations.
- Integrating service learning into the dermatology residency program curriculum enhances trainees’ cultural sensitivity and encourages the prioritization of skin health equity.
- Service learning will help bridge the gap in access to dermatologic care for patients in medically marginalized communities.
Tinted Sunscreens: Consumer Preferences Based on Light, Medium, and Dark Skin Tones
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Practice Points
- Visible light has been shown to increase tyrosinase activity and induce immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.
- The formulation of sunscreens with iron oxides and pigmentary titanium dioxide are a safe and effective way to protect against high-energy visible light, especially when combined with zinc oxide.
- Physicians should be aware of sunscreen characteristics that patients like and dislike to tailor recommendations that are appropriate for each individual to enhance adherence.
- Cosmetic elegance and tone compatibility are the most important criteria for individuals seeking tinted sunscreens.