User login
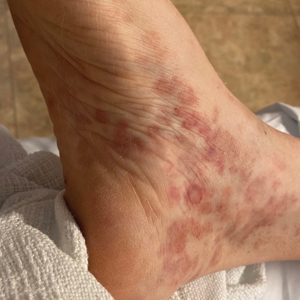
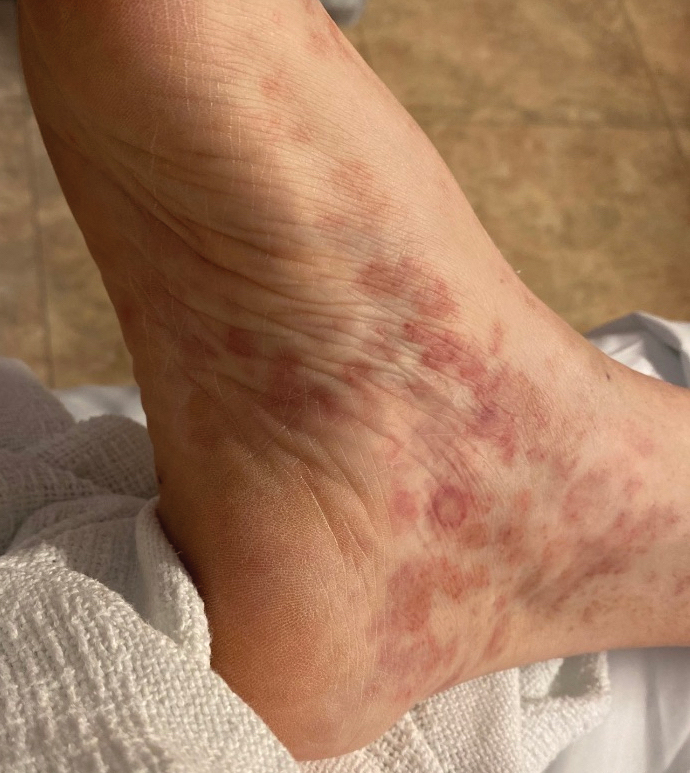
Nonscaly Red-Brown Macules on the Feet and Ankles
THE DIAGNOSIS: Secondary Syphilis
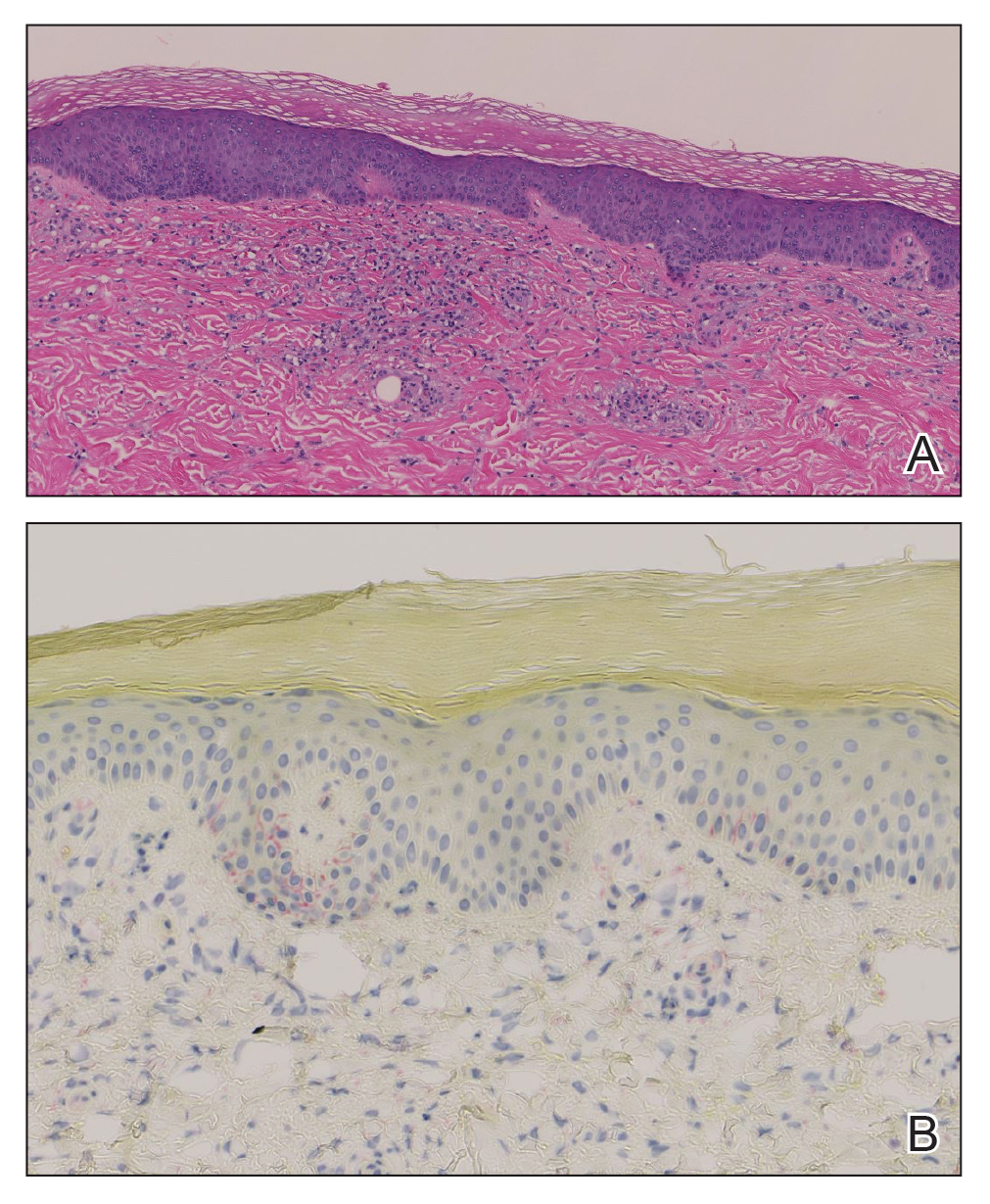
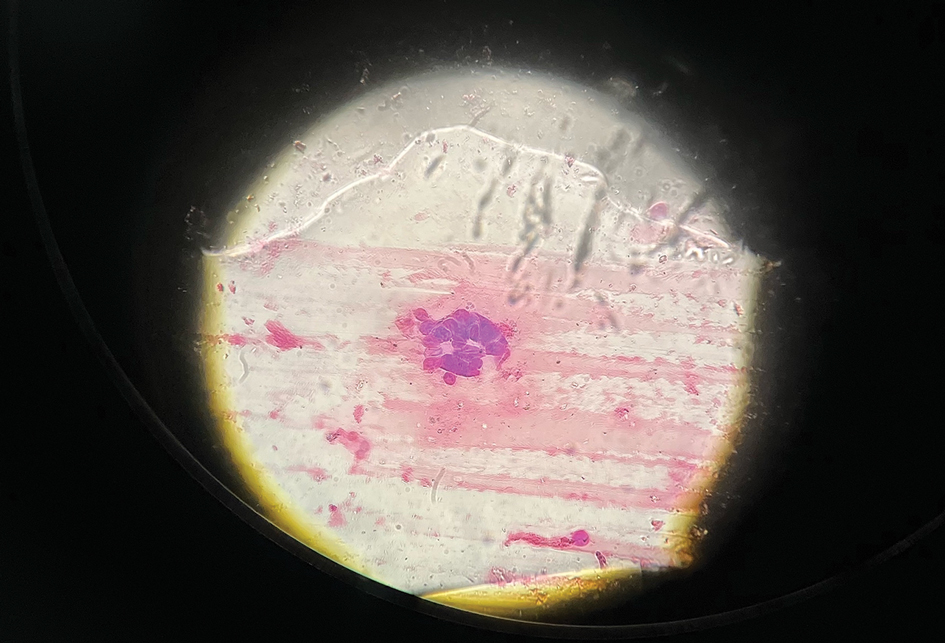
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.
Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
A 59-year-old man presented with a nontender nonpruritic rash on the feet of 2 days’ duration. The patient had a several-year history of granulomatosis with polyangiitis (GPA) and was taking methotrexate and prednisone. The rash appeared suddenly—first on the right foot and then on the left foot—and was preceded by 1 week of worsening polyarthralgia, most notably in the ankles. He denied any fever, chills, sore throat, or weight loss. His typical GPA symptoms included inflammatory arthritis, scleritis, leukocytoclastic vasculitis, and sinonasal and renal involvement. He recently experienced exacerbation of inflammatory arthritis that required an increase in the prednisone dosage (from 40 mg to 60 mg daily), but there were no other GPA symptoms. He had a history of multiple female sexual partners but no known history of HIV and no recent testing for sexually transmitted infections. Hepatitis C antibody testing performed 5 years earlier was nonreactive. He denied any illicit drug use, recent travel, sick contacts, or new medications.
Dermatologic examination revealed nonscaly, clustered, red-brown macules, some with central clearing, on the medial and lateral aspects of the feet and ankles with a few faint copper-colored macules on the palms and soles. The ankles had full range of motion with no edema or effusion. There were no oral or genital lesions. The remainder of the skin examination was normal. Punch biopsies of skin on the left foot were obtained for histopathology and direct immunofluorescence.
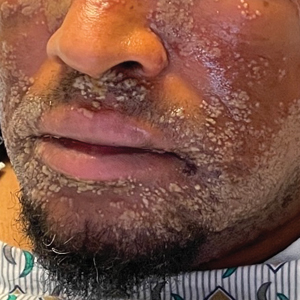
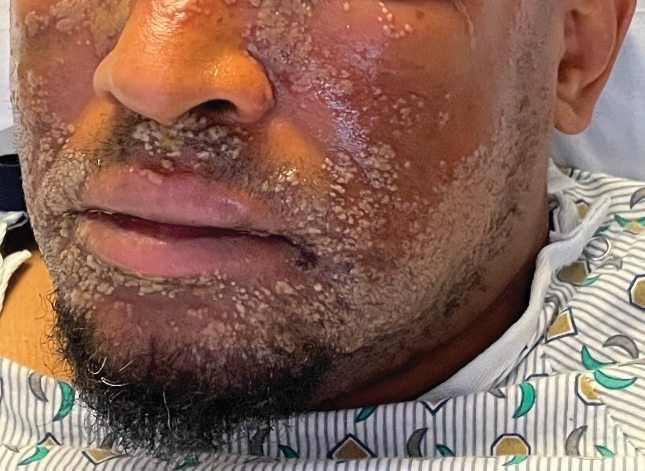
Pustular Eruption on the Face
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.
Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
A 52-year-old man developed a sudden eruption of small pustules on background erythema and edema covering the forehead, nasal bridge, periorbital region, cheeks, and perioral region on day 3 of hospitalization in the intensive care unit for management of septic shock secondary to a complicated urinary tract infection. He had a medical history of benign prostatic hyperplasia, sarcoidosis, and atopic dermatitis. He initially presented to the emergency department with fever, chills, and dysuria of 2 days’ duration. Because he received ceftriaxone, vancomycin, ciprofloxacin, and tamsulosin while hospitalized for the infection, the primary medical team suspected a drug reaction and empirically started applying hydrocortisone cream 2.5%. The rash continued to spread over the ensuing day, prompting a dermatology consultation to rule out a drug eruption and to help guide further management. The patient was in substantial distress and pain. Physical examination revealed numerous discrete and confluent monomorphic pustules on background erythema with faint collarettes of scale covering most of the face. Substantial periorbital and facial edema forced the eyes closed. There was no mucous membrane involvement. A review of systems was negative for dyspnea and dysphagia, and the rash was not present elsewhere on the body. Ophthalmologic evaluation revealed no ocular involvement or vision changes. Laboratory studies demonstrated neutrophilia (17.27×109 cells/L [reference range, 2.0–6.9×109 cells/L]). The eosinophil count, blood urea nitrogen/creatinine, and liver function tests were within reference range.
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
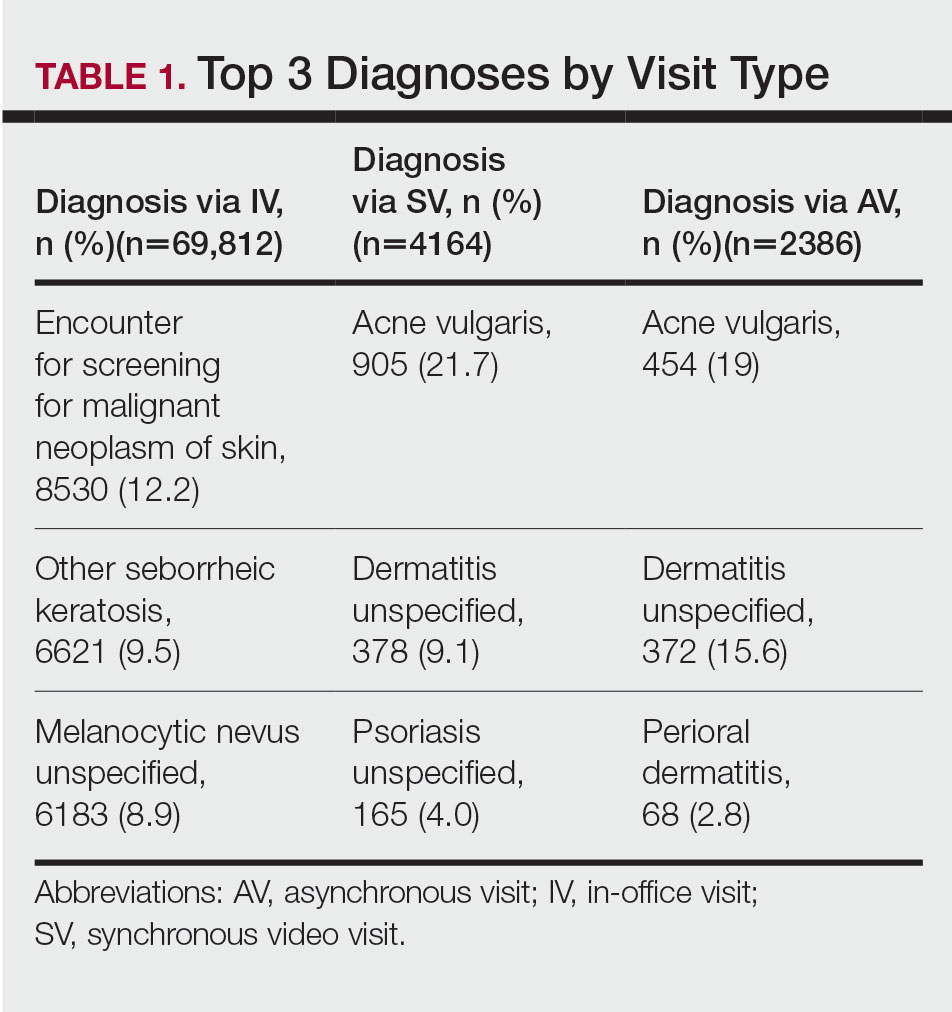
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
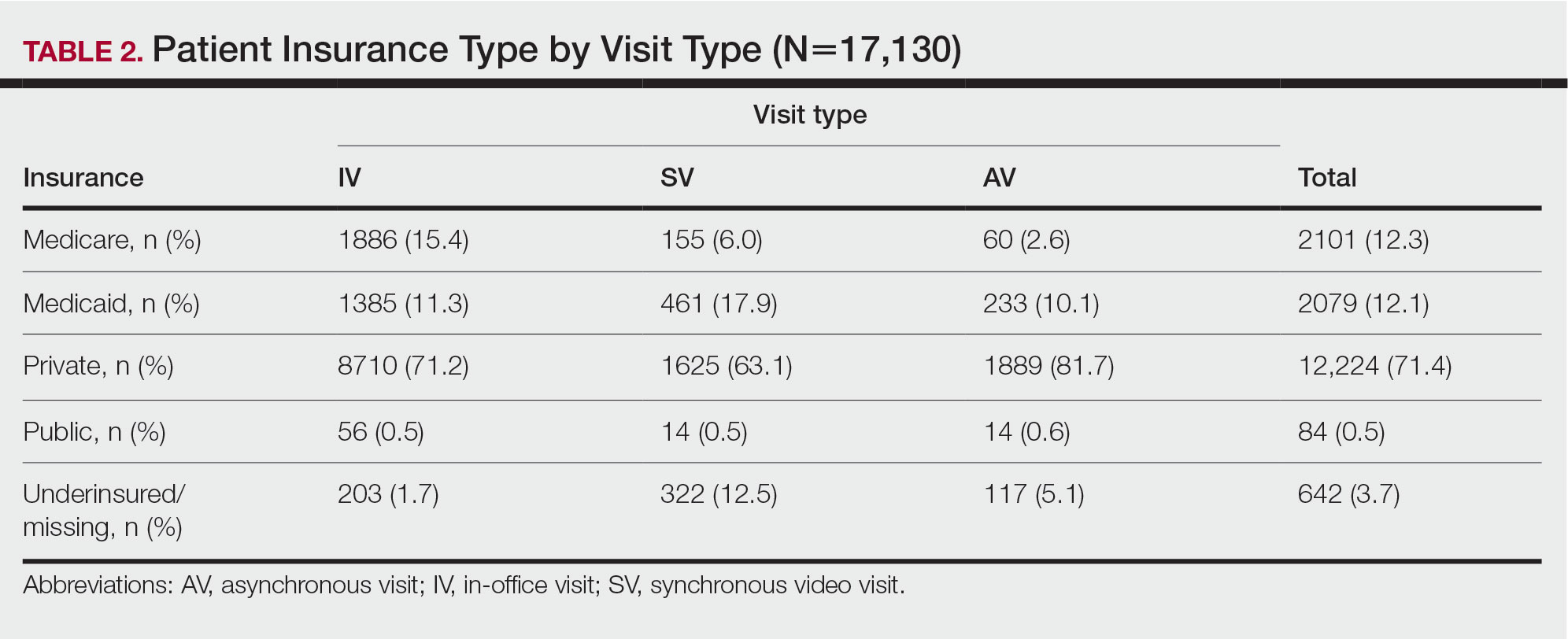
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
Perceptions of Community Service in Dermatology Residency Training Programs: A Survey-Based Study of Program Directors, Residents, and Recent Dermatology Residency Graduates
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.
Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).
Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.
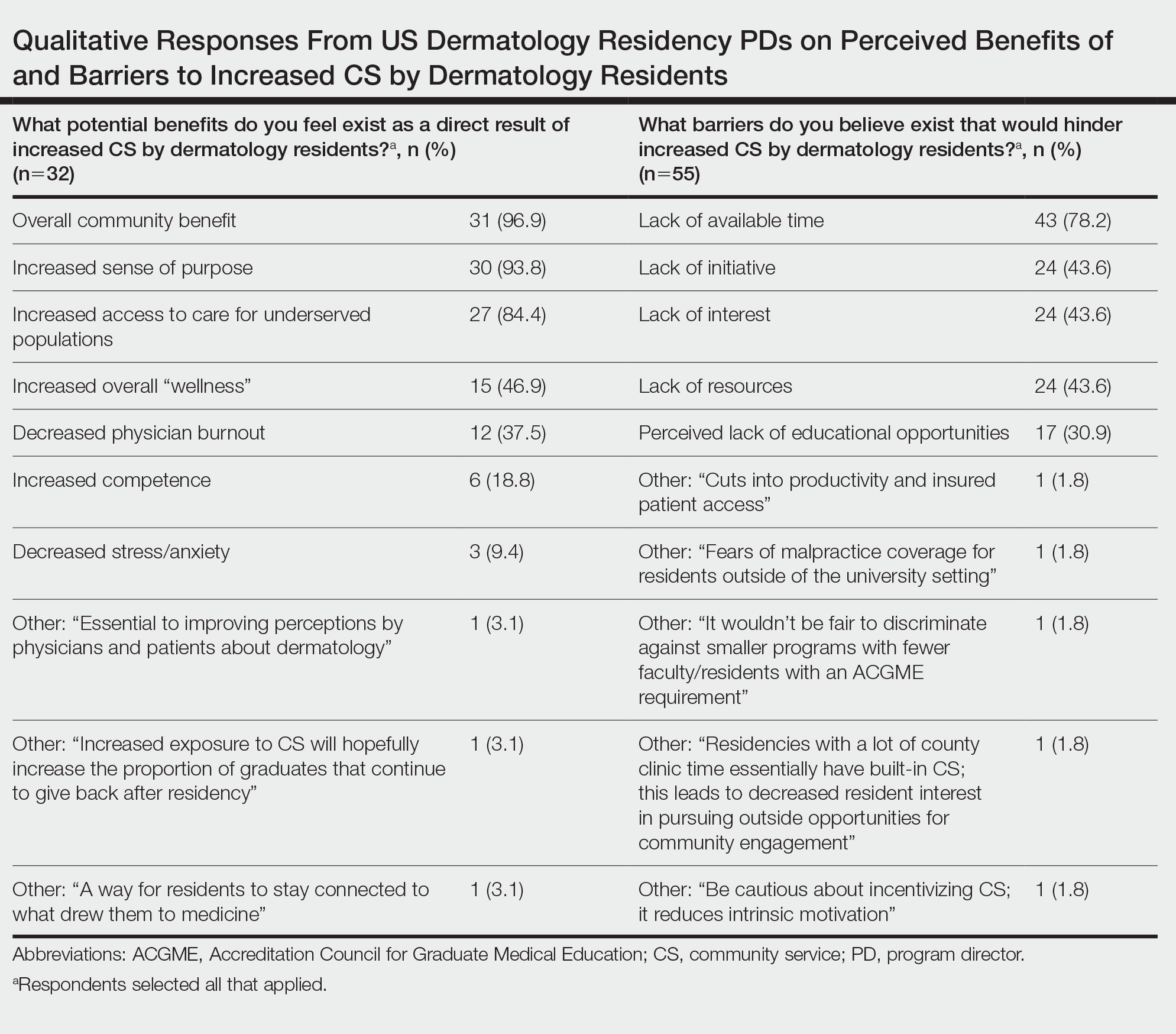
Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Participation of dermatology residents in service-learning experiences increases awareness of health disparities and social factors impacting dermatologic care and promotes a lifelong commitment to serving vulnerable populations.
- Integrating service learning into the dermatology residency program curriculum enhances trainees’ cultural sensitivity and encourages the prioritization of skin health equity.
- Service learning will help bridge the gap in access to dermatologic care for patients in medically marginalized communities.
The Importance of Service Learning in Dermatology Residency: An Actionable Approach to Improve Resident Education and Skin Health Equity
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Practice Points
- In 2019, nearly 30 million Americans did not have health insurance. Dermatologists in the United States should be cognizant of the challenges faced by underserved patients when accessing dermatologic care.
- Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive learning experience, meet societal needs, and fulfill Accreditation Council for Graduate Medical Education requirements.
- Actionable methods to increase service learning in dermatology residency training include volunteering in community service programs offered by national dermatology organizations, implementing service-learning projects, and partnering with free and federally funded community practices.
- Dermatology residents who participate in service learning will help increase access to equitable dermatologic care and experience practicing in settings with limited resources.