User login
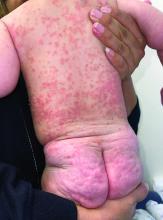
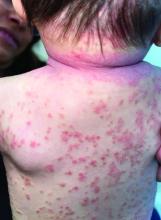
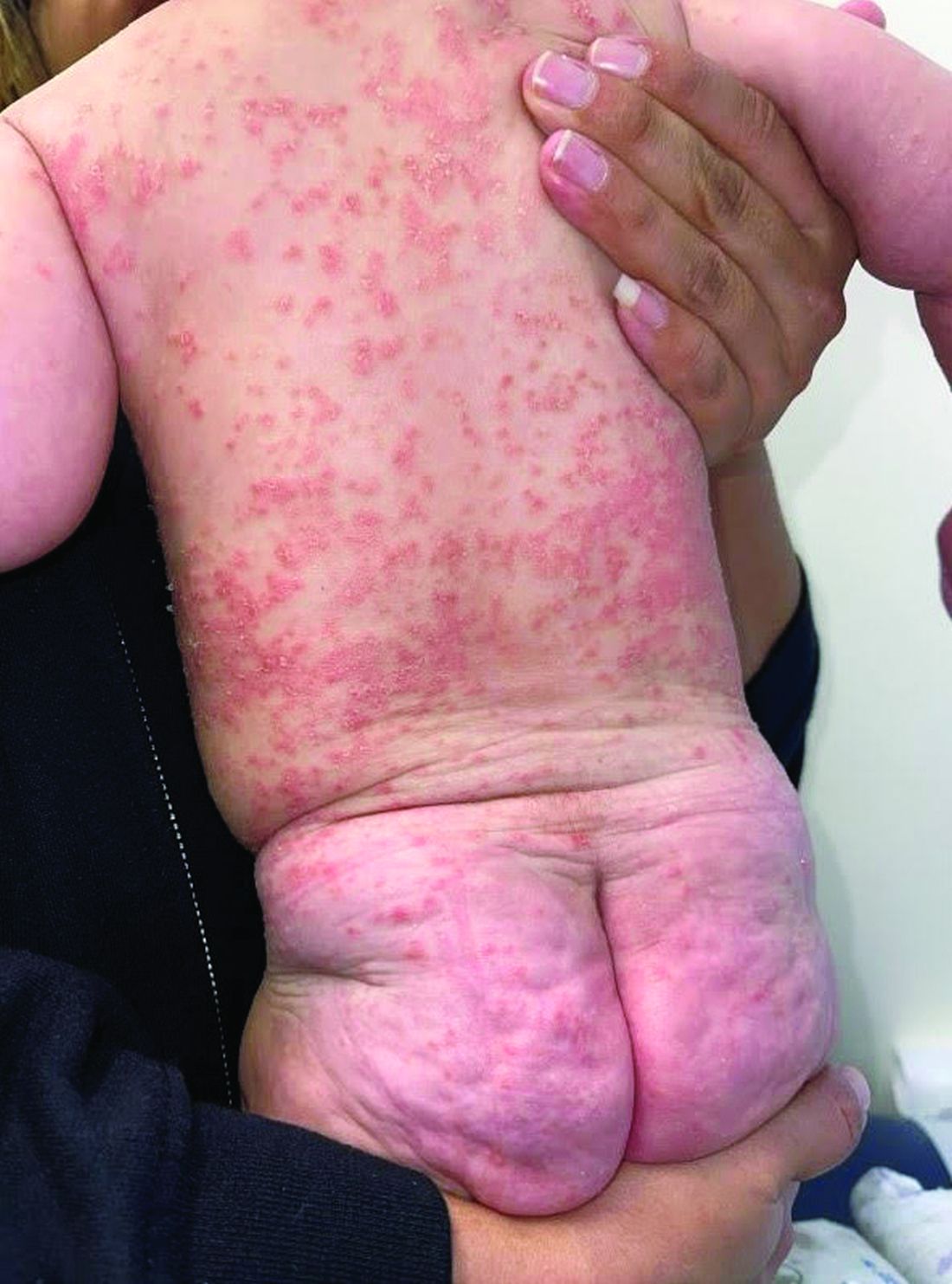
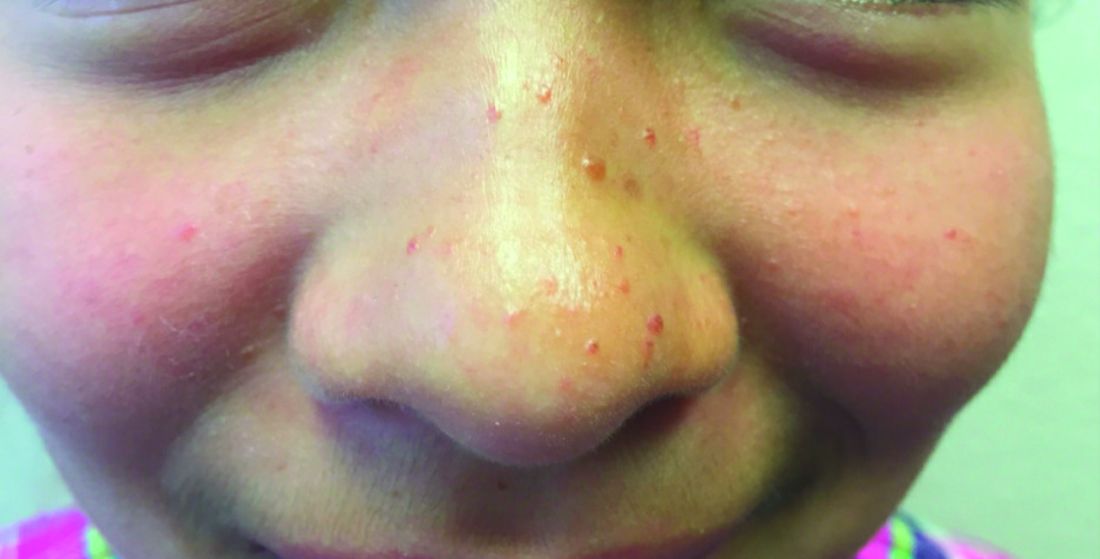
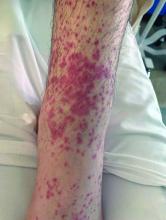
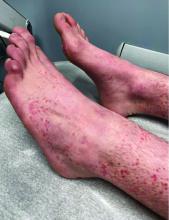
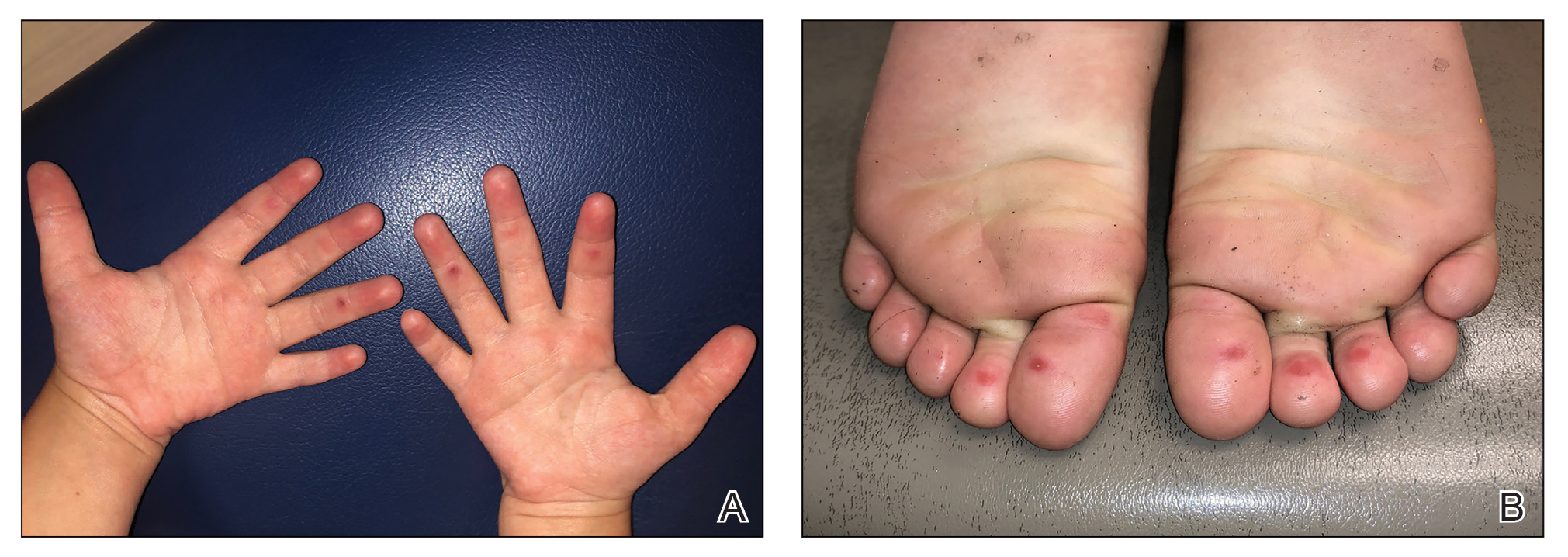
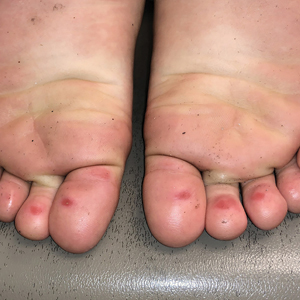
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.
What is the diagnosis?
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
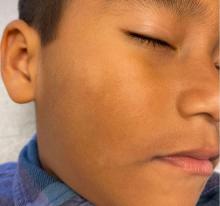
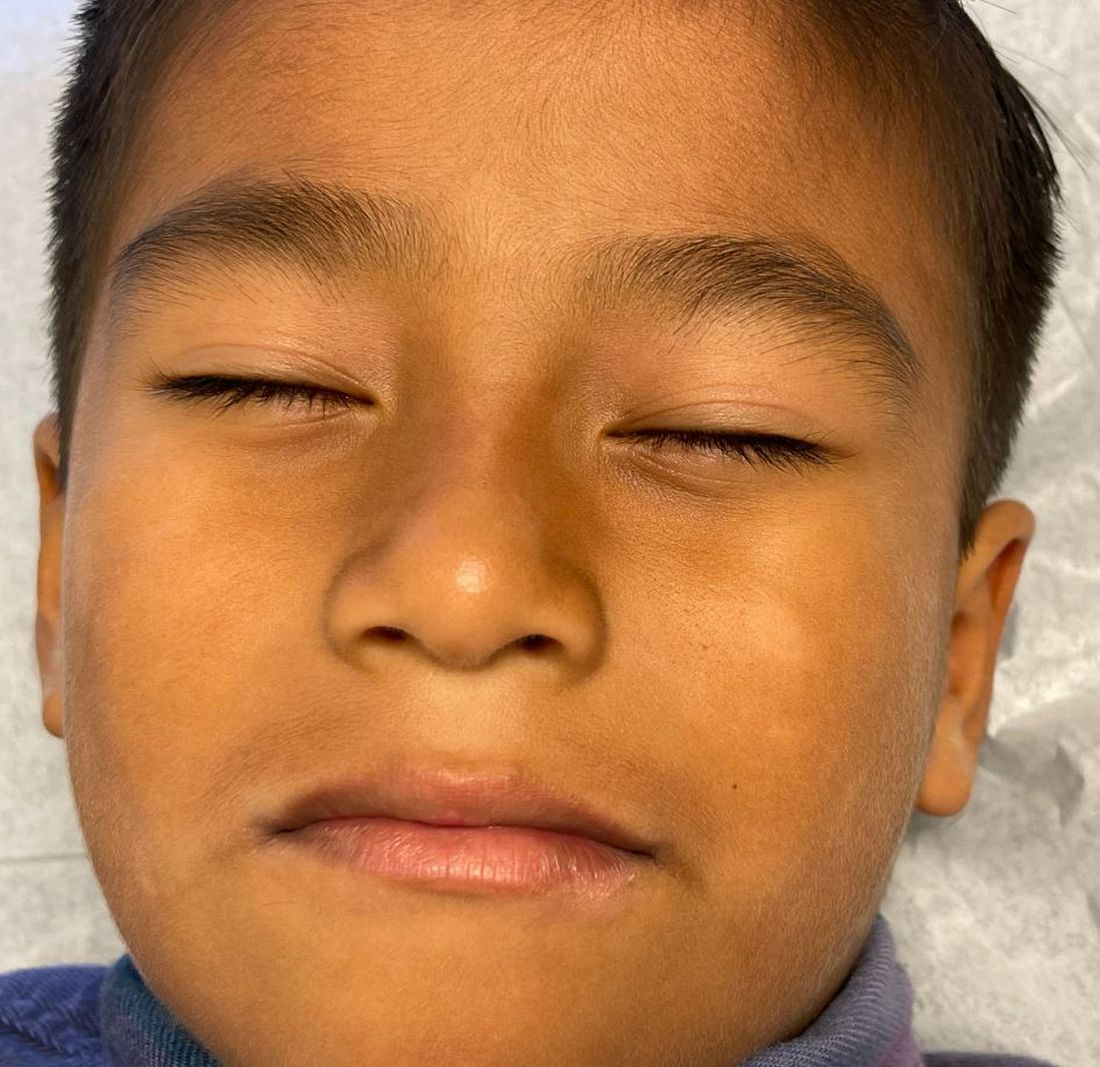
The lesions were asymptomatic, and the review of systems was otherwise negative.
Physical examination revealed multiple poorly defined thin hypopigmented patches with a bilateral distribution, mostly on the cheeks.
The patches had focal superficial nonadherent thin white scales and were mildly rough to the touch. The rest of the physical exam was unremarkable, including no active eczematous lesions on the trunk or extremities.
What's the diagnosis?
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
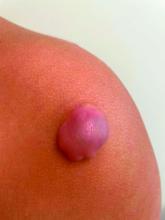
On physical exam there was a well-circumscribed skin-colored nodule measuring 3.1 x 3 cm that was tender on palpation. The nodule was mobile, with a firm, stony feel, and no punctum was visualized. Transillumination revealed a subtle bluish hue within the nodule.
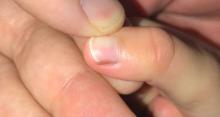
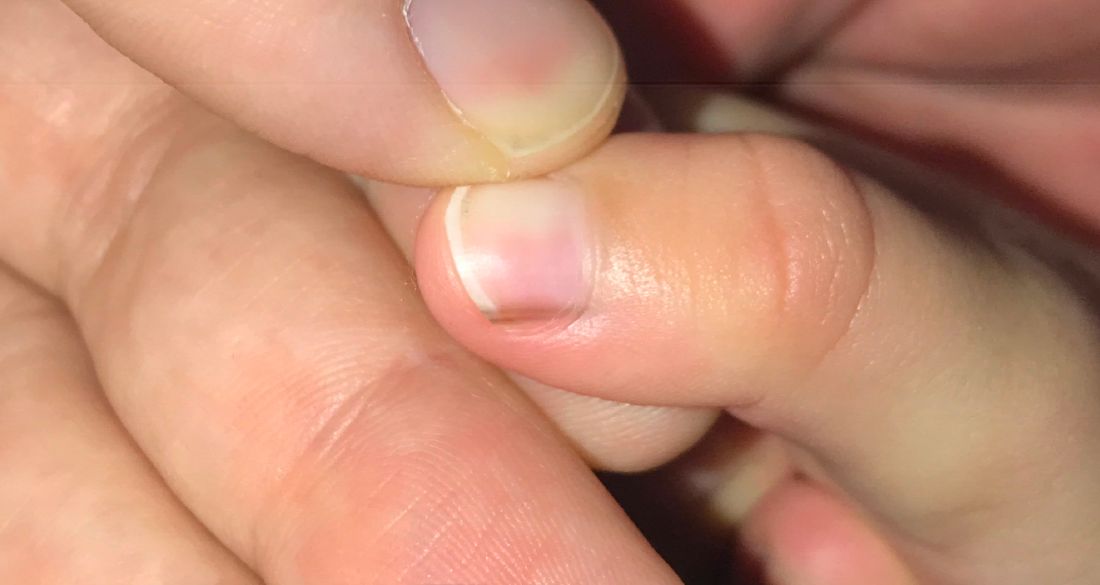
A toddler presents with a dark line on a fingernail
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Examination findings reveal a 2-mm brown longitudinal band on the radial aspect of the right thumbnail that does not extend into the proximal or lateral nailfolds. The rest of the skin and nail exam is unremarkable.
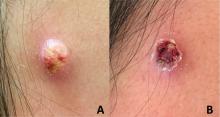
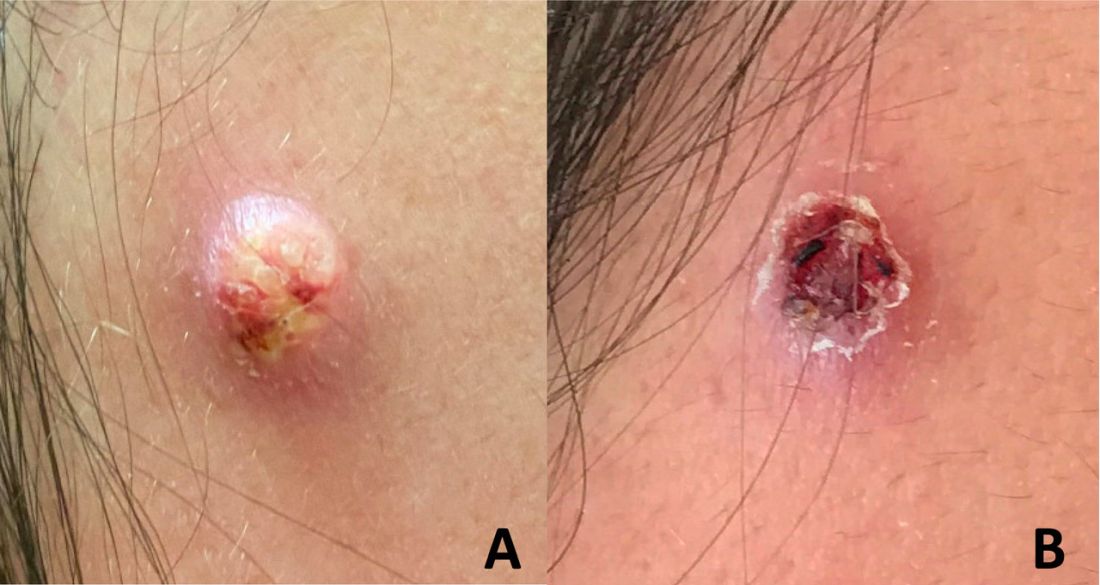
An adolescent male presents with an eroded bump on the temple
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
Past, Present, and Future of Pediatric Atopic Dermatitis Management
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
PRACTICE POINTS
- Pediatric atopic dermatitis (AD) therapeutics have rapidly evolved over the last decade and dermatologists should be aware of new tools in their treatment arsenal.
- New topical nonsteroidal agents serve as useful alternatives to topical corticosteroids through mitigating adverse effects from current standard therapy and potentially simplifying topical regimens.
- Monoclonal antibodies and Janus kinase inhibitors are part of an important set of new systemic therapeutics for pediatric AD.
- Long-term data on these new therapeutics is required to better understand their impact on pediatric AD comorbidities and impact on the longitudinal disease course.
A toddler presents with patchy hair loss
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Given the history of sudden hair loss, with the exam revealing a well-circumscribed patch of focal alopecia without cutaneous inflammation, hairs with a narrow base and broad distal shaft, the diagnosis is alopecia areata (AA).
Alopecia areata (AA) is a nonscarring alopecia, within a set of diseases characterized by the preservation of hair follicles and therefore the potential for future hair regrowth.1 AA is believed to be caused by a breakdown of the immune-privileged nature of hair follicles, resulting in T-lymphocytes targeting the hair follicle directly, shifting follicles to early catagen or telogen phase, but sparing follicular stem cells, thereby allowing the follicle to regenerate in the future.1-3 Risk factors include family history of AA, thyroid disorders, as well as iron and vitamin D deficiency.4,5 It characteristically presents with focal, well-demarcated patches of hair loss in the scalp, typically with background skin normal to slightly pink.3,6 Exam can show “exclamation point” hairs consisting of hairs that are narrow at their base and wide at the distal end.3,7 Patients may also exhibit eyebrow and eyelash loss as well as nail changes including nail pitting and splitting.8 Diagnosis is typically made clinically but is supported by a positive hair pull test, where hairs are pulled from the periphery of an alopecic lesion; the presence of greater than 10% of hairs plucked from the scalp indicates a positive result.9,10
What’s the differential diagnosis?
The differential diagnosis of AA includes other nonscarring alopecias such as trichotillomania and telogen effluvium. Other possible diagnoses include lichen planopilaris and tinea capitis.
Trichotillomania results in irregularly bordered hair loss and broken hairs of different lengths because of an internal urge to remove one’s hair, resulting in nonscarring alopecia. It can be associated with obsessive-compulsive disorder, anxiety, or other body-altering behaviors like skin picking and nail biting (characterized as body-focused repetitive behavior disorders). Treatments include reassurance and education, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs. Toddlers can engage in hair pulling behavior and trichotillomania can be difficult to differentiate from AA. However, the absence of broken hairs of varying lengths makes trichotillomania less likely in this patient.
Telogen effluvium is another form of nonscarring alopecia that presents as diffuse hair thinning across the entire scalp in response to acute psychological or physiological stress, hormonal changes, certain medications, systemic illness, or nutritional deficiency. The timing between the triggering event and hair loss can vary from weeks to months. Diagnosis requires detailed history-taking and may include evaluation for endocrinologic hair thinning (e.g. thyroid function tests) to identify reversible causes. Treatment involves directing therapy to the underlying etiology and most cases of telogen effluvium are self-limited. The presence of a well-circumscribed patch of hair loss in this patient makes AA more likely.
Lichen planopilaris (LPP) is a scarring, irreversible alopecia caused by T-lymphocytes attacking follicular hair stem cells. It is characterized by hair loss, pruritus, burning pain, scalp scaling, and multifocal scarring. Exam shows patches of alopecia with loss of follicular ostia centrally and perifollicular scale and erythema at the borders. Diagnosis is aided by biopsy of the affected scalp. Treatment of LPP requires the use of potent and superpotent topical corticosteroids and intralesional corticosteroids to decrease scalp inflammation and prevent further progression. The presence of follicular ostia and absence of perifollicular scale in this patient makes LPP highly unlikely.
Tinea capitis is a fungal infection of the scalp caused by dermatophytes including Trychophyton tonsurans and Microsporum canis. It presents with patches of alopecia with overlying scale and broken hairs and can have associated cervical and occipital lymphadenopathy. Diagnosis can involve skin scraping and KOH prep to visualize branching hyphae as well as fungal culture to identify the causative organism. Because dermatophytes in tinea capitis invade hair follicles, topical antifungals are ineffective because of their lack of penetration. Therefore, systemic antifungals including oral terbinafine and griseofulvin are considered first-line agents for treatment.
What’s the management plan?
The diagnosis of AA is usually a clinical one, though assessment of alternative diagnoses is appropriate dependent on signs and symptoms. Workup of AA can include thyroid studies because of the association with autoimmune thyroid disease, though studies suggest limited screening benefits in children.11 Given its variable and unpredictable course, management can include “watchful waiting” because of its potential for spontaneous remission.6 For limited patchy loss, active treatment with mid to superpotent topical steroids or intralesional triamcinolone acetonide in older children and adolescents is reasonable.12 Other treatment options include topical or low-dose oral minoxidil and immunotherapy with diphenylcyclopropenone or squaric acid (inducing an allergic contact dermatitis).12 Management of therapies for more extensive AA is evolving, with ongoing studies of oral JAK-inhibitors and biologic agents.12,13
Our patient was started on topical fluocinonide 0.05% solution and achieved good disease control and hair regrowth over the course of 3 months.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology at the university and Rady Children’s Hospital. They had no disclosures.
References
1. Bernardez C et al. Actas Dermosifiliogr. 2015;106(3):158-67.
2. Rajabi F et al. Br J Dermatol. 2018;179(5):1033-48.
3. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):1-12.
4. Lee S et al. J Am Acad Dermatol. 2019;80(2):466-77 e16.
5. MacLean KJ and Tidman MJ. Practitioner. 2013;257(1764):29-32, 3.
6. Pratt CH et al. Nat Rev Dis Primers. 2017;3:17011.
7. Gilhar A et al. N Engl J Med. 2012;366(16):1515-25.
8. Wyrwich KW et al. Am J Clin Dermatol. 2020;21(5):725-32.
9. Spano F and Donovan JC. Can Fam Physician. 2015;61(9):751-5.
10. Mounsey AL and Reed SW. Am Fam Physician. 2009;80(4):356-62.
11. Hordinsky MK. J Investig Dermatol Symp Proc. 2015;17(2):44-6.
12. Strazzulla LC et al. J Am Acad Dermatol. 2018;78(1):15-24.
13. Zhou C et al. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Examination findings of the scalp demonstrate a well-circumscribed alopecic patch on the vertex scalp without erythema or scale. Closer inspection of the patch with magnification or 'dermoscopy' reveals hair follicle ostia and hairs that are broader distally and narrower at their base. Nails and rest of the skin exam are unremarkable.
A 7-year-old with red bumps on her nose
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
A 7-year-old female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks.
A 7-year-old, previously healthy female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks. She has no significant medical history aside from a wart on her hand that was recently frozen with liquid nitrogen and resolved. She denied pruritus, bumps, or skin changes elsewhere on the body. The patient was prescribed tretinoin 0.1% cream applied nightly for several months without response.
What is the diagnosis?
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Not COVID Toes: Pool Palms and Feet in Pediatric Patients
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.