User login
Participation in Work and Sport Following Reverse and Total Shoulder Arthroplasty
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
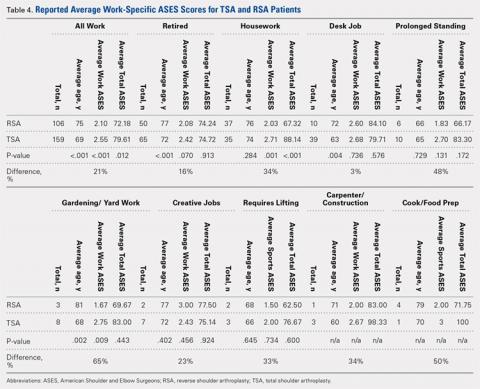
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
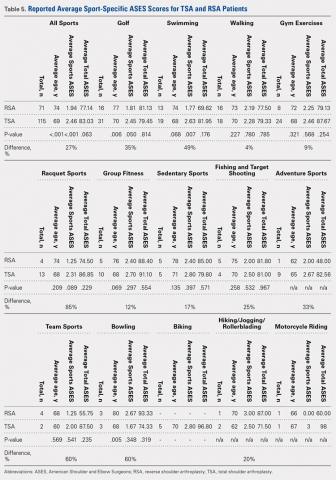
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
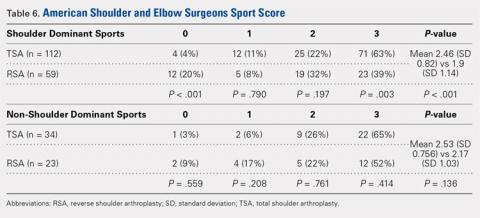
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
TAKE-HOME POINTS
- Both anatomic (TSA) and reverse shoulder arthroplasty (RSA) allow for the participation in work and sports.
- TSA patients report easier overall ability to participate in sports, specifically golf and swimming.
- For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
- TSA patients report easier overall ability to return to work-related activities, specifically housework and gardening.
- TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients.
Short-Term Projected Use of Reverse Total Shoulder Arthroplasty in Proximal Humerus Fracture Cases Recorded in Humana’s National Private-Payer Database
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
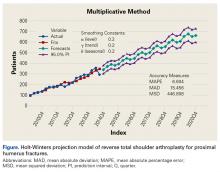
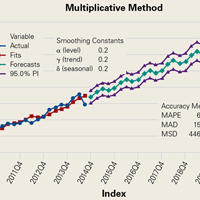
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
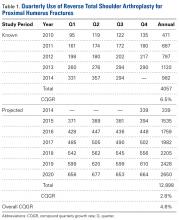
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
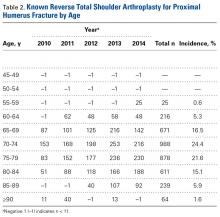
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.