User login
Short-Term Projected Use of Reverse Total Shoulder Arthroplasty in Proximal Humerus Fracture Cases Recorded in Humana’s National Private-Payer Database
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
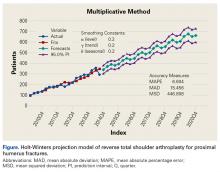
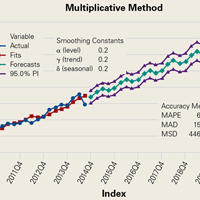
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
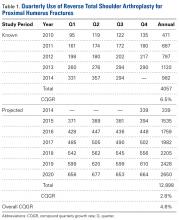
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
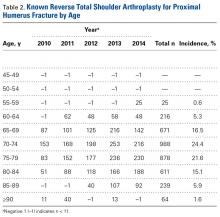
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- RTSA is projected to triple by 2020.
- RTSA for fracture indication anticipates a 4.9% compound quarterly growth rate.
- RTSA is gaining in popularity likely due to unpredictable results of hemiarthroplasty in select patients.
Reverse total shoulder arthroplasty (RTSA) is an accepted treatment option for the pain and dysfunction associated with glenohumeral arthritis and severe rotator cuff pathology.1-3 Recently, it has been gaining acceptance as an alternative to hemiarthroplasty (HA) and open reduction and internal fixation (ORIF) in the surgical management of complex proximal humerus fractures (PHFs) in elderly patients.4-6 The advantages of RTSA over other PHF treatment options include a lower revision rate and superior range of motion.4,5
PHF remains one of the most common fracture pathologies in the United States.7 Given the country’s aging patient population, the popularity of RTSA likely will continue to increase.4-6 The release of supercomputer data from individual private-payer insurance providers provides an opportunity to investigate trends in the surgical management of PHFs and to formulate models for predicting use. In this study, we used a large private-payer database to analyze these trends over the period 2010 to 2014 and project RTSA use through 2020.
Methods
We used PearlDiver’s supercomputer application to search the Humana private-payer database to retrospectively identify cases of PHF treated with the index procedure of RTSA. PearlDiver, a publicly available national database compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996), compiles private-payer records submitted by Humana. These records represent 100% of the orthopedics-related payer records within the dataset. The database includes International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedural Terminology (CPT) codes from 2007 to 2014.
RTSA cases were identified by ICD-9 codes 81.80 and 81.88 and CPT code 23472. PHFs were identified by ICD-9, Clinical Modification (ICD-9-CM) codes 812.00, 812.01, 812.02, 812.03, 812.09, 812.10, 812.11, 812.12, 812.13, 812.19, and 812.20. Holt-Winters quarterly (Q) projection analysis was performed on the RTSA-PHF data from Q1-2010 through Q4-2020 (Figure).
Results
For the known study period Q1-2010 through Q3-2014, our search yielded 46,106 PHF cases, 4057 (8.8%) of which were surgically treated with RTSAs (Table 1).
Age-based subgroup analysis revealed RTSA was performed primarily in the older-than-65 years patient population, with the highest percentage in the 70-to-74 years age group (24.4%), followed by the 75-to-79 years age group (21.6%) (Table 2).
Discussion
Use of RTSA for the management of complex PHFs has increased tremendously over the past several years. The primary results of our study showed an upward trend in RTSA use in the Humana population. CQGR was 6.5% from Q1-2010 through Q3-2014 (the number of RTSAs increased to 294 from 95). Based on the Holt-Winters projection analysis, CQGR was projected to be 2.8% through 2020 (339 RTSAs in Q4-2014 increasing to 664 RTSAs in Q4-2020), resulting in an overall 10-year CQGR of 4.6%.
Recent studies have shown RTSA to be a viable alternative to HA in patients with PHFs. It has been suggested that RTSAs may have more reliable clinical outcomes without a comparative increase in complication rates.1,8,9 HA has been associated with unpredictable motion, higher complication rates, and high rates of unsatisfactory results in patients older than 65 years.10-12 In addition, studies have found that, compared with HA and ORIF, RTSA produces superior range of motion.8,9 The reliability of clinical outcomes in the early transition to use of RTSA for complex fractures suggests that use of RTSA for PHF management is trending upward. Results of the present study showed a steady increase in RTSA use. This trend is further supported by a recent study finding on national trends in RTSA use in PHF cases: 12.3% annual growth during the period 2000 to 2008.6Our study results showed a continued steady quarterly increase in use of RTSA for PHFs, projected to triple by Q4-2020 (Table 1). The increasing popularity of RTSA may be attributable to its better clinical outcomes and to the procedural instruction given to newly trained orthopedic surgeons during residency. A recent study found a substantial increase in the use of RTSA for PHFs—from 2% in 2005 to 38% in 2012—among newly trained orthopedic surgeons.13 Another possible driver of the increase is cost. Although RTSA implant costs are often a multiple of the costs of other treatment options, different findings were reported in 2 recent studies that used quality-adjusted life-years (QALY) to determine RTSA cost-effectiveness. Coe and colleagues14 compared RTSA with HA and found RTSA to be cost-effective but highly dependent on implant cost. They determined that an implant cost of over $13,000 put RTSA cost-effectiveness at just under $100,000 QALY, whereas an implant cost of under $7000 brought QALY down to under $50,000. Renfree and colleagues15 used the same QALY benchmark but found RTSA to be at the highly cost-effective threshold of under $25,000 QALY.
Current literature recommends RTSA be performed primarily for elderly patients.1,2,16,17 Guery and colleagues2 suggested limiting RTSA to patients who are older than 70 years and have low functional demands. In 2 studies of RTSA use in complex humeral fractures, Gallinet and colleagues16,18 found an increased rate of scapular notching in younger patients and recommended restricting RTSA to patients 70 years or older. PHFs in patients older than 70 years often have more complex fracture patterns and poor-quality bone, which makes fracture healing more challenging in HA and ORIF settings. As tuberosity healing is crucial to functional outcomes of surgically treated PHFs, RTSA has been advanced as a more reliable option in patients in whom tuberosity healing is expected to be unreliable. The present study’s finding that 68.5% of the RTSA patients in the Humana population were older than 70 years further supports the literature’s emphasis on reserving RTSA for patients over 70 years.
This study had its limitations. The PearlDiver database depends on accurate ICD-9 and CPT coding, and there was potential for reporting bias. In addition, a new, specific ICD-9 code for RTSA was introduced in 2010 and may not have been immediately used; data reported during this time could have been affected. Furthermore, the data were primarily represented by a single private-payer organization (Humana) and therefore may not have fully encapsulated the entire US trend. Projection in this study did not account for US Census–predicted population growth and therefore may have underestimated the true projected use of RTSA for PHFs.
This study benefited from the completeness of the data used. PearlDiver represents 100% of Humana claims data, providing a large patient population for analysis and capturing data as recent as 2014. To our knowledge, no other large database studies have used such up-to-date data.
Conclusion
RTSA is becoming an increasingly popular treatment option for PHFs. Modest overall quarterly growth in use of RTSA for PHFs (CQGR, 4.6%) is predicted through Q4-2020. Number of RTSAs performed for PHF management is projected to more than triple by 2020.
Am J Orthop. 2017;46(1):E28-E31. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.
1. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
2. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(8):1742-1747.
3. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
4. Anakwenze OA, Zoller S, Ahmad CS, Levine WN. Reverse shoulder arthroplasty for acute proximal humerus fractures: a systematic review. J Shoulder Elbow Surg. 2014;23(4):e73-e80.
5. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426.
6. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
7. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Jones KJ, Dines DM, Gulotta L, Dines JS. Management of proximal humerus fractures utilizing reverse total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2013;6(1):63-70.
10. Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202-209.
11. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412.
12. Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4(2):81-86.
13. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367.
14. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
15. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661.
16. Gallinet D, Adam A, Gasse N, Rochet S, Obert L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(1):38-44.
17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
18. Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48-55.