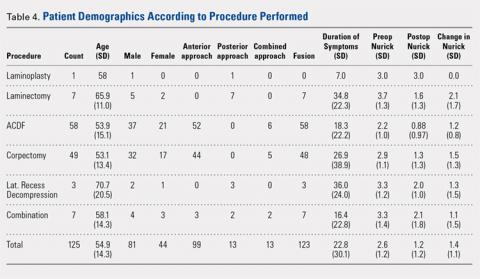
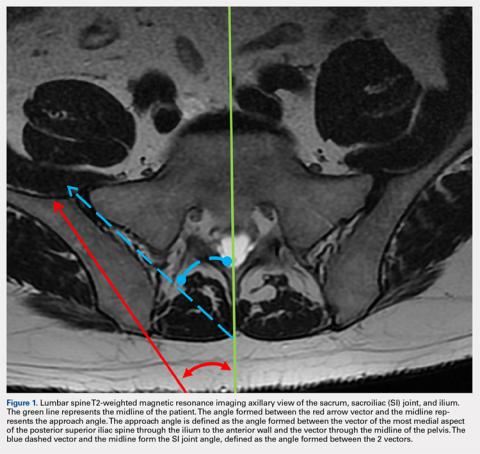
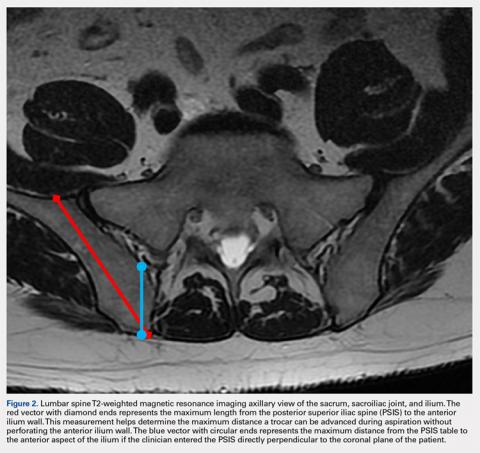
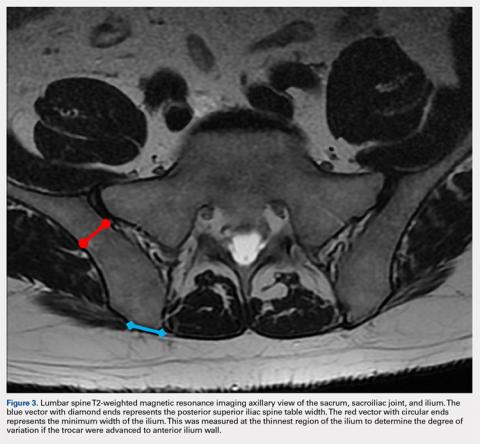
User login
Diabetes/Weight Loss Med Linked to Repeat Spinal Surgery
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
POISE-3 backs wider use of tranexamic acid in noncardiac surgery
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Simple prevention strategies can lessen postoperative delirium after orthopedic surgery
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
A new study has found that and a prevention program can help improve staff education and outcomes.
“In an aging society, it is very important to develop and implement a strategy for POD prevention to ensure that aging patients are treated as safely and effectively as possible,” wrote Jung-Yeon Choi of Seoul (South Korea) National University Bundang Hospital and coauthors. The study was published in BMC Geriatrics.
To determine how to better identify and treat high-risk patients for POD after orthopedic surgery, the researchers led a retrospective cohort study that included an intervention group of participants who were aged at least 65 years (n = 275) and a control group from a year prior (n = 274). Patients in the intervention group had their risk of delirium assessed and categorized using a simple screening tool, and those deemed at risk were entered into a multicomponent delirium prevention program.
Of the 275 patients in the intervention group, 144 required screening for delirium. Ninety-nine were classified as low risk, 29 were classified as high risk, and 16 missed the screening. Fifty-three additional patients were classified as high risk because they were aged 80 years or older. During the study, 17 participants experienced POD, 16 of whom were classified as high risk. In regard to estimating POD risk, the sensitivity and specificity of the delirium screening tool were 94.1% and 72.7%, respectively. Incidence rates of POD were 10.2% in the control group and 6.2% in the intervention group.
The authors noted their study’s limitations, including its design as a retrospective review of medical records rather than a prospective randomized controlled trial. In addition, because it was conducted in just one teaching hospital, they deemed it “not possible to determine the generalizability and long-term effect of our findings.”
The authors reported no conflicts of interest.
SOURCE: Choi JY et al. BMC Geriatr. 2019 Oct 26. doi: 10.1186/s12877-019-1303-z.
FROM BMC GERIATRICS
Nerve transfer improves function after spinal cord injury
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
FROM LANCET
Fragility Fractures: Diagnosis and Treatment
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
TAKE-HOME POINTS
- 3 million people sustain fragility fractures annually, and nearly 30% die within a year of the fracture.
- The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.
- The World Health Organization has developed a set of T-core criteria to diagnose osteoporosis in postmenopausal women: a score >–1 is normal; <–1 but >–2.5 signifies osteopenia; <–2.5 denotes osteoporosis; and <–2.5 with fragility fracture indicates severe osteoporosis.
- The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children. The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender. Z scores <–2.0 indicate low BMD for chronological age. A Z score > –2.0 is considered within the expected range for age.
- After an initial fragility fracture, the risk for additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture.26
The Effect of Age on the Benefits of Early Decompression for Cervical Spondylotic Myelopathy
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
TAKE-HOME POINTS
- Decompression of cervical myelopathy within 24 months of symptom onset results in greater functional improvement compared to delayed decompression.
- The improvement with respect to time is more significant for patients older than 65 years compared to younger patients.
- Duration of symptoms does not seem to influence the severity of the preoperative Nurick score.
- Preoperative severity of symptoms is related to postoperative outcomes.
- Other significant predictors of worse outcomes include tobacco use, diabetes, and number of levels fused.
Nearly one-quarter of presurgery patients already using opioids
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
FROM JAMA SURGERY
Key clinical point: Preoperative opioid use is prevalent in patients who are having spinal surgery and have depression.
Major finding:
Study details: An observational study of 34,186 surgical patients in the University of Michigan Health system.
Disclosures: The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
Source: Hilliard P E et al. JAMA Surg. 2018 Jul 11;. doi:10.1001/jamasurg.2018.2102.
An MRI Analysis of the Pelvis to Determine the Ideal Method for Ultrasound-Guided Bone Marrow Aspiration from the Iliac Crest
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
ABSTRACT
Use of mesenchymal stem cells from bone marrow has gained significant popularity. The iliac crest has been determined to be an effective site for harvesting mesenchymal stem cells. Review of the literature reveals that multiple techniques are used to harvest bone marrow aspirate from the iliac crest, but the descriptions are based on the experience of various authors as opposed to studied anatomy. A safe, reliable, and reproducible method for aspiration has yet to be studied and described. We hypothesized that there would be an ideal angle and distance for aspiration that would be the safest, most consistent, and most reliable. Using magnetic resonance imaging (MRI), we reviewed 26 total lumbar spine MRI scans (13 males, 13 females) and found that an angle of 24° should be used when entering the most medial aspect of the posterior superior iliac spine (PSIS) and that this angle did not differ between the sexes. The distance that the trocar can advance after entry before hitting the anterior ilium wall varied significantly between males and females, being 7.53 cm in males and 6.74 cm in females. In addition, the size of the PSIS table was significantly different between males and females (1.20 cm and 0.96 cm, respectively). No other significant differences in the measurements gathered were found. Using the data gleaned from this study, we developed an aspiration technique. This method uses ultrasound to determine the location of the PSIS and the entry point on the PSIS. This contrasts with most techniques that use landmark palpation, which is known to be unreliable and inaccurate. The described technique for aspiration from the PSIS is safe, reliable, reproducible, and substantiated by data.
The iliac crest is an effective site for harvesting bone marrow stem cells. It allows for easy access and is superficial in most individuals, allowing for a relatively quick and simple procedure. Use of mesenchymal stem cells (MSCs) for treatment of orthopedic injuries has grown recently. Whereas overall use has increased, review of the literature reveals very few techniques for iliac crest aspiration,1 but these are not based on anatomic relationships or studies. Hernigou and colleagues2,3 attempted to quantitatively evaluate potential “sectors” allowing for safe aspiration using cadaver and computed tomographic reconstruction imaging. We used magnetic resonance imaging (MRI) to analyze aspiration parameters. Owing to the ilium’s anatomy, improper positioning or aspiration technique during aspiration can result in serious injury.2,4-6 We hypothesized that there is an ideal angle and positioning for bone marrow aspiration from the posterior superior iliac spine (PSIS) that is safe, consistent, and reproducible. Although most aspiration techniques use landmark palpation, this is unreliable and inaccurate, especially when compared with ultrasound-guided injections7-16 and procedures.9,12,17-19 We describe our technique using ultrasound to visualize patient anatomy and accurately determine anatomic entry with the trocar.
METHODS
MRI scans of 26 patients (13 males, 13 females) were reviewed to determine average angles and distances. Axial T2-weighted views of the lumbar spine were used in all analyses. The sacroiliac (SI) joint angle was defined as the angle formed between the vector through the midline of the pelvis and the vector that is parallel to the SI joint. The approach angle was defined as the angle formed between the vector of the most medial aspect of the PSIS through the ilium to the anterior wall and the vector through the midline of the pelvis (Figure 1).
Continue to: For the 13 males, the mean SI joint...
RESULTS
The results are reported in the Table.
Table. Measurements of Patients Taken on Axial T2-Weighted Views of Lumbosacral MRI Scansa
Patient | SI Joint Angle (°) | Approach Angle (°) | PSIS Table Width (cm) | PSIS to Anterior Ilium Wall (cm) | Perpendicular Distance PSIS to Anterior Joint (cm) | Post Ilium Wall to SI Joint Width (cm) |
Males | ||||||
1 | 28.80 | 19.50 | 1.24 | 8.80 | 4.16 | 1.52 |
2 | 31.80 | 27.60 | 1.70 | 7.89 | 3.49 | 1.02 |
3 | 33.70 | 27.70 | 1.12 | 8.14 | 3.15 | 1.28 |
4 | 23.70 | 26.40 | 0.95 | 6.66 | 3.22 | 0.65 |
5 | 35.90 | 28.40 | 0.84 | 7.60 | 2.57 | 0.95 |
6 | 33.80 | 29.30 | 1.20 | 7.73 | 2.34 | 0.90 |
7 | 30.30 | 21.20 | 1.36 | 8.44 | 3.95 | 1.18 |
8 | 34.50 | 20.40 | 1.53 | 7.08 | 3.98 | 1.56 |
9 | 28.70 | 24.00 | 1.34 | 8.19 | 3.51 | 1.31 |
10 | 22.40 | 20.10 | 1.37 | 7.30 | 3.87 | 1.28 |
11 | 33.60 | 20.80 | 0.88 | 6.43 | 3.26 | 0.94 |
12 | 48.50 | 31.00 | 1.15 | 6.69 | 2.97 | 1.38 |
13 | 20.20 | 20.90 | 0.94 | 6.95 | 3.79 | 1.05 |
Averages | 31.22 | 24.41 | 1.20 | 7.53 | 3.40 | 1.16 |
Standard Deviation | 7.18 | 4.11 | 0.26 | 0.75 | 0.56 | 0.26 |
Females | ||||||
14 | 22.80 | 23.20 | 1.54 | 7.21 | 3.45 | 1.39 |
15 | 33.30 | 21.40 | 1.09 | 7.26 | 3.57 | 0.98 |
16 | 19.70 | 15.60 | 0.78 | 8.32 | 3.76 | 0.86 |
17 | 17.50 | 15.60 | 0.61 | 7.57 | 3.37 | 1.03 |
18 | 48.20 | 26.60 | 0.94 | 6.62 | 3.16 | 0.71 |
19 | 38.20 | 28.30 | 0.90 | 6.32 | 2.23 | 0.91 |
20 | 44.50 | 31.70 | 0.99 | 6.19 | 3.06 | 0.76 |
21 | 24.10 | 18.00 | 0.92 | 6.99 | 3.23 | 0.71 |
22 | 17.20 | 14.80 | 0.81 | 6.00 | 2.81 | 1.13 |
23 | 42.00 | 38.50 | 1.00 | 5.33 | 2.47 | 1.42 |
24 | 32.00 | 25.50 | 0.98 | 6.01 | 2.79 | 1.21 |
25 | 24.70 | 24.80 | 0.87 | 6.09 | 2.79 | 1.02 |
26 | 19.80 | 22.30 | 1.04 | 7.71 | 2.37 | 1.36 |
Averages | 29.54 | 23.56 | 0.96 | 6.74 | 3.00 | 1.04 |
Standard Deviation | 10.84 | 6.88 | 0.21 | 0.85 | 0.48 | 0.25 |
All patients Averages | 30.38 | 23.98 | 1.08 | 7.14 | 3.20 | 1.10 |
Standard Deviation | 9.05 | 5.57 | 0.26 | 0.88 | 0.55 | 0.26 |
aStatistical significance is denoted as P < .02.
Abbreviations: MRI, magnetic resonance imaging; PSIS, posterior iliac spine; SI, sacroiliac.
For the 13 males, the mean SI joint angle was 31.22° ± 7.18° (range, 20.20° to 48.50°). The mean approach angle was 24.41° ± 4.11° (range, 19.50° to 31.00°). The mean PSIS table width was 1.20 cm ± 0.26 cm (range, 0.84 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.53 cm ± 0.75 cm (range, 6.43 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.40 cm ± 0.56 cm (range, 2.34 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.16 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
For the 13 females, the mean SI joint angle was 29.54° ± 10.84° (range, 17.20° to 48.20°). The mean approach angle was 23.56° ± 6.88° (range, 14.80° to 38.50°). The mean PSIS table width was 0.96 cm ± 0.21 cm (range, 0.61 cm to 1.54 cm). The mean distance from the PSIS to the anterior ilium wall was 6.74 cm ± 0.85 cm (range, 5.33 cm to 8.32 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.00 cm ± 0.48 cm (range, 2.23 cm to 3.76 cm). The mean minimum width of the ilium to the SI joint was 1.04 cm ± 0.25 cm (range, 0.71 cm to 1.42 cm).
For the 26 total patients, the mean SI joint angle was 30.38° ± 9.05° (range, 17.20° to 48.50°). The mean approach angle was 23.98° ± 5.57° (range, 14.80° to 38.50°). The mean PSIS table width was 1.08 cm ± 0.26 cm (range, 0.61 cm to 1.70 cm). The mean distance from the PSIS to the anterior ilium wall was 7.14 cm ± 0.88 cm (range, 5.33 cm to 8.80 cm). The mean perpendicular distance from the PSIS table to the anterior ilium was 3.20 cm ± 0.55 cm (range, 2.23 cm to 4.16 cm). The mean minimum width of the ilium to the SI joint was 1.10 cm ± 0.26 cm (range, 0.65 cm to 1.56 cm).
There was a statistically significant difference between the male and female groups for the maximum distance the trocar can be advanced from the PSIS to the anterior ilium wall (P < .02), and a statistically significant difference for the PSIS table width (P < .02). There were no significant differences between the male and female groups for the approach angle, the SI joint angle, the perpendicular distance from the PSIS to the anterior ilium, and the minimum width of the ilium to the SI joint.
Continue to: The patient is brought to the procedure...
TECHNIQUE: ILIAC CREST (PSIS) BONE MARROW ASPIRATION
The patient is brought to the procedure room and placed in a prone position. The donor site is prepared and draped in the usual sterile manner. Ultrasound is used to identify the median sacral crest in a short-axis view. The probe is then moved laterally to identify the PSIS (Figures 4A, 4B).
The crosshairs on the ultrasound probe are used to mark the center lines of each plane. The central point marks the location of the PSIS. Alternatively, an in-plane technique can be used to place a spinal needle on the exact entry point on the PSIS. Once the PSIS and entry point are identified, the site is blocked with 10 mL of 0.5% ropivacaine.
Prior to introduction of the trocar, all instrumentation is primed with heparin and syringes are prepped with anticoagulant citrate dextrose solution, solution A. A stab incision is made at the site. The trocar is placed at the entry point, which should be centered in a superior-inferior plane and at the most medial point of the PSIS. Starting with the trocar vertical, the trocar is angled laterally 24° by dropping the hand medially toward the midline. No angulation cephalad or caudad is necessary, but cephalad must be avoided so as not to skive superiorly. This angle, which is recommended for both males and females, allows for the greatest distance the trocar can travel in bone before hitting the anterior ilium wall. A standard deviation of 5.57° is present, which should be considered. Steady pressure should be applied with a slight twisting motion on the PSIS. If advancement of the trocar is too difficult, a mallet or drill can be used to assist in penetration.
With the trocar advanced into the bone 1 cm, the trocar needle is removed while the cannula remains in place. The syringe is attached to the top of the cannula. The syringe plunger is pulled back to aspirate 20 mL of bone marrow. The cannula and syringe assembly are advanced 2 cm farther into the bone to allow for aspiration of a new location within the bone marrow cavity, and 20 mL of bone marrow are again aspirated. This is done a final time, advancing the trocar another 2 cm and aspirating a final 20 mL of bone marrow. The entire process should yield roughly 60 mL of bone marrow from one side. If desired, the same process can be repeated for the contralateral PSIS to yield a total of 120 mL of bone marrow from the 2 sites.
Based on our data, the average distance to the anterior ilium wall was 7 cm, but the shortest distance noted in this study was 5 cm. On the basis of the data presented, this technique allows for safe advancement based on even the shortest measured distance, without fear of puncturing the anterior ilium wall. Perforation could damage the femoral nerve and the internal or external iliac artery or vein that lie anterior to the ilium.
Continue to: We hypothesized that there...
DISCUSSION
We hypothesized that there would be an optimal angle of entry and maximal safe distance the trocar could advance through the ilium when aspirating. Because male and female pelvic anatomy differs, we also hypothesized that there would be differences in distance and size measurements for males and females. Our results supported our hypothesis that there is an ideal approach angle. The results also showed that the maximum distance the trocar can advance and the width of the PSIS table differ significantly between males and females.
Although pelvic anatomy differs between males and females, there should be an ideal entry angle that would allow maximum advancement into the ilium without perforating the anterior wall, which we defined as the approach angle. In our comparison of 26 MRI scans, we found that the approach angle did not differ significantly between the 2 groups (13 males, 13 females). This allows clinicians to enter the PSIS at roughly 24° medial to the parasagittal line, maximizing the space before puncturing into the anterior pelvis in either males or females.
If clinicians were to enter perpendicular to the patient’s PSIS, they would, on average, be able to advance only 3.20 cm before encountering the SI joint. When entering at 24° as we recommend, the average distance increases to 7.14 cm. Although the angle did not differ significantly, there was a significant difference between males and females in the length from the PSIS to the anterior wall, with males having 7.53 cm distance and females 6.74 cm. This is an important measurement because if the anterior ilium wall is punctured, the femoral nerve and the common, internal and external iliac arteries and veins could be damaged, resulting in retroperitoneal hemorrhage.
A fatality in 2001 in the United Kingdom led to a national audit of bone marrow aspiration and biopsies.4-6 Although these procedures were done primarily for patients with cancer, hemorrhagic events were the most frequent and serious events. This audit led to the identification of many risk factors. Bain4-6 conducted reviews of bone marrow aspirations and biopsies in the United Kingdom from 2002 to 2004. Of a total of 53,088 procedures conducted during that time frame, 48 (0.09%) adverse events occurred, with 29 (0.05%) being hemorrhagic events. Although infrequent, hemorrhagic adverse events represent significant morbidity. Reviews such as those conducted by Bain4-6 highlight the importance of a study that helps determine the optimal parameters for aspiration to ensure safety and reliability.
Hernigou and colleagues2,3 conducted studies analyzing different “sectors” in an attempt to develop a safe aspiration technique. They found that obese patients were at higher risk, and some sites of aspiration (sectors 1, 4, 5) had increased risk for perforation and damage to surrounding structures. Their sector 6, which incorporated the entirety of the PSIS table, was considered the safest, most reliable site for trocar introduction.2,3 Hernigou and colleagues,2 in comparing the bone mass of the sectors, also noted that sector 6 has the greatest bone thickness close to the entry point, making it the most favorable site. The PSIS is not just a point; it is more a “table.” The PSIS can be palpated posteriorly, but this is inaccurate and unreliable, particularly in larger individuals. The PSIS table can be identified on ultrasound before introducing the trocar, which is a more reliable method of landmark identification than palpation guidance, just as in ultrasound-guided injections7-16 and procedures.9,12,17-19
Continue to: If the PSIS is not accurately...
If the PSIS is not accurately identified, penetration laterally will result in entering the ilium wing, where it is quite narrow. We found the distance between the posterior ilium wall and the SI joint to be only 1.10 cm wide (Figure 3); we defined this area as the narrow corridor. Superior and lateral entry could damage the superior cluneal nerves coming over the iliac crest, which are located 6 cm lateral to the SI joint. Inferior and lateral entry 6 cm below the PSIS could reach the greater sciatic foramen, damaging the sacral plexus and superior gluteal artery and vein. If the entry slips above the PSIS over the pelvis, the trocar could enter the retroperitoneal space and damage the femoral nerve and common iliac artery and vein, leading to a retroperitoneal hemorrhage.4-6,20
MSCs are found as perivascular cells and lie in the cortices of bones.21 Following the approach angle and directed line from the PSIS to the anterior ilium wall described in this study (Figures 1 and 2), the trocar would pass through the narrow corridor as it advances farther into the ilium. The minimum width of this corridor was measured in this study and, on average, was 1.10 cm wide from cortex to cortex (Figure 3). As the bone marrow is aspirated from this narrow corridor, the clinician is gathering MSCs from both the lateral and medial cortices of the ilium. By aspirating from a greater surface area of the cortices, it is believed that this will increase the total collection of MSCs.
CONCLUSION
Although there are reports in the literature that describe techniques for bone marrow aspiration from the iliac crest, the techniques are very general and vague regarding the ideal angles and methods. Studies have attempted to quantify the safest entry sites for aspiration but have not detailed ideal parameters for collection. Blind aspiration from the iliac crest can have serious implications if adverse events occur, and thus there is a need for a safe and reliable method of aspiration from the iliac crest. Ultrasound guidance to identify anatomy, as opposed to palpation guidance, ensures anatomic placement of the trocar while minimizing the risk of aspiration. Based on the measurements gathered in this study, an optimal angle of entry and safe distance of penetration have been identified. Using our data and relevant literature, we developed a technique for a safe, consistent, and reliable method of bone marrow aspiration out of the iliac crest.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
1. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441-e445. doi:10.1016/j.eats.2016.10.024.
2. Hernigou J, Alves A, Homma Y, Guissou I, Hernigou P. Anatomy of the ilium for bone marrow aspiration: map of sectors and implication for safe trocar placement. Int Orthop. 2014;38(12):2585-2590. doi:10.1007/s00264-014-2353-7.
3. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384. doi:10.1007/s00264-014-2343-9.
4. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol. 2005;58(4):406-408. doi:10.1136/jcp.2004.022178.
5. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26(5):315-318. doi:10.1111/j.1365-2257.2004.00630.x.
6. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91(9):1293-1294.
7. Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging. 2012;7:89-95. doi:10.2147/CIA.S29265.
8. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282. doi:10.1016/j.arthro.2005.12.019.
9. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
10. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84-A(9):1522-1527.
11. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
12. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
13. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
14. Sibbit WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892-1902. doi:10.3899/jrheum.090013.
15. Smith J, Brault JS, Rizzo M, Sayeed YA, Finnoff JT. Accuracy of sonographically guided and palpation guided scaphotrapeziotrapezoid joint injections. J Ultrasound Med. 2011;30(11):1509-1515. doi:10.7863/jum.2011.30.11.1509.
16. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
17. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
18. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
19. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Submitted.
20. Jamaludin WFW, Mukari SAM, Wahid SFA. Retroperitoneal hemorrhage associated with bone marrow trephine biopsy. Am J Case Rep. 2013;14:489-493. doi:10.12659/AJCR.889274.
21. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35-42. doi:10.1038/nm.3028.
TAKE-HOME POINTS
- There is an ideal angle and distance for optimization of a bone marrow harvest from the iliac crest.
- Ultrasound is a reliable technology that allows clinicians to accurately and consistently identify the PSIS and avoid neurovascular structures.
- This safe, reliable bone marrow aspiration technique can lower the risk of serious potential complications.
- The ideal angle does not differ significantly between sexes, but the safe distance a clinician can advance does.
- The PSIS should be considered a “table” as opposed to a protuberance.