Therese Borden is the editor of CHEST Physician. After 20 years of research, writing, and editing in the field of international development and economics, she began working in the field of medical editing and has held a variety of editorial positions with the company. She holds a PhD in International Economics from American University, Washington, and a BA in history from the University of Washington, Seattle.
News

Sleepless in the pandemic
- Author:
- Therese Borden
Overexposure to media COVID-19 content may contribute to emotional distress and sleeplessness.
News
‘Collateral damage’: COVID-19 threatens patients with COPD
- Author:
- Therese Borden
Patients presenting at the ED with acute exacerbations of COPD could experience diagnostic and treatment delays.
News
Trial undertaken to better predict pulmonary hypertension prognosis
- Author:
- Therese Borden
The study is designed to establish the clinical utility of daily activity tracking in patients with pulmonary...
News

Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
- Author:
- Therese Borden
There were relatively few cases in children and they had relatively mild disease.
News
Second U.S. coronavirus patient confirmed
- Author:
- Therese Borden
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care provider.
News
Washington state patient is first U.S. case of novel coronavirus
- Author:
- Therese Borden
The outbreak began at an animal and meat market in China and now has spread to at least four other countries....
News
Two new cases of coronavirus pneumonia in Thailand, Japan
- Author:
- Therese Borden
The new cases of 2019-nCoV were identified in travelers from the affected province in China.
News

Mystery pneumonia in China has health officials on alert
- Author:
- Therese Borden
There are no reports of person-to-person transmission or health care worker infection of this pneumonia.
News
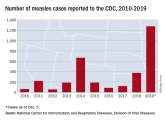
The measles comeback of 2019
- Author:
- Therese Borden
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York.
News

EVALI outbreak ongoing, but new cases decline
- Author:
- Therese Borden
Since the week of Sept. 15, 2019, hospitalized cases of EVALI have steadily declined.
News
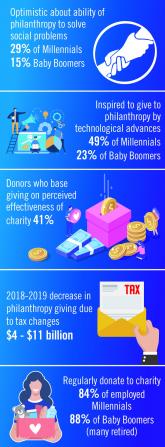
Environmental Scan: Drivers of philanthropy
- Author:
- Therese Borden
Charitable giving, foundation support, and grants touch the lives of millions of patients and have an impact across all fields of practice of...
News

Vaping-linked lung injury: 2,172 cases, 42 deaths
- Author:
- Therese Borden
Forty-two deaths from EVALI have been confirmed in 24 states and the District of Columbia.
News
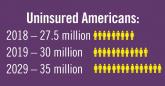
Environmental Scan: Drivers of social, political and environmental change
- Author:
- Therese Borden
Spiraling costs of medical care, consumer activism around health care delivery, an aging population, and growing evidence of climate change are...
News

CDC identifies probable culprit in vaping lung injuries
- Author:
- Therese Borden
In addition, obtaining vaping devices from informal sources, such as from a dealer, off the street, or from a friend, was more likely to be linked...
News
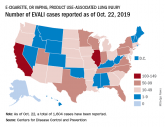
Vaping-linked lung injury cases near 1,900
- Author:
- Therese Borden
Thirty-seven deaths from vaping-associated lung injuries have been reported in 24 states and the District of Columbia.
