User login
Therese Borden is the editor of CHEST Physician. After 20 years of research, writing, and editing in the field of international development and economics, she began working in the field of medical editing and has held a variety of editorial positions with the company. She holds a PhD in International Economics from American University, Washington, and a BA in history from the University of Washington, Seattle.
Environmental Scan: Drivers of philanthropy
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
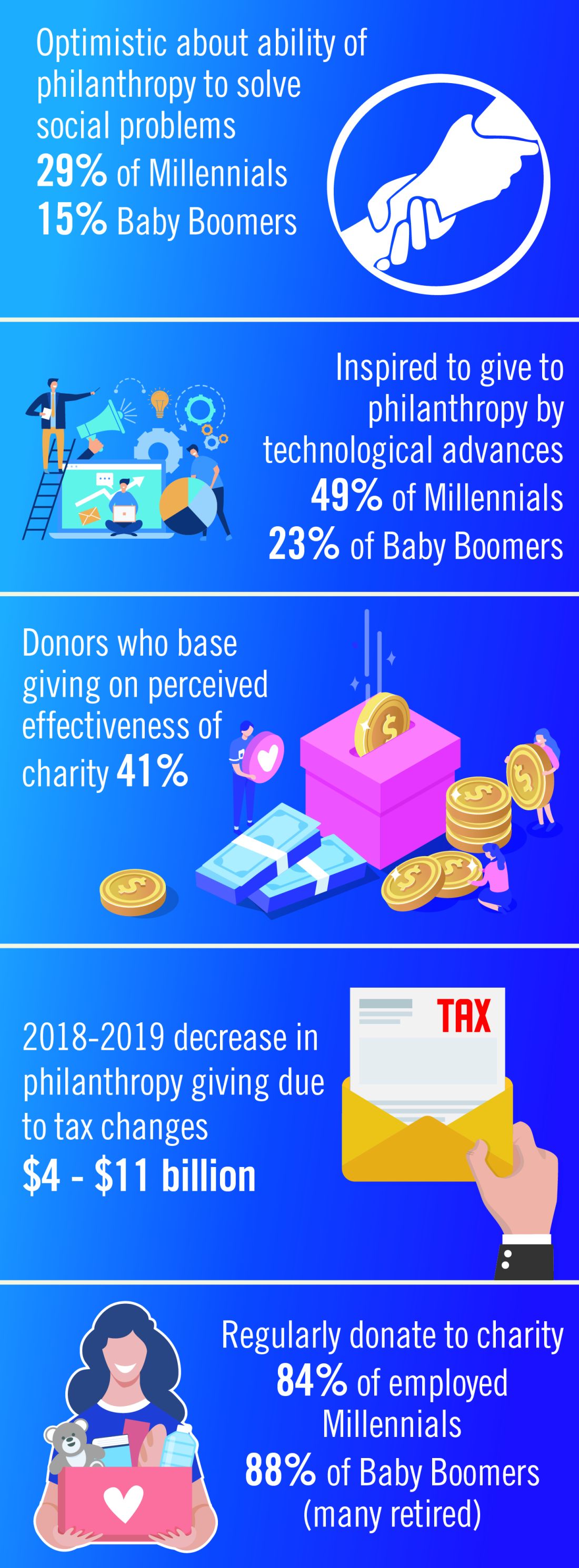
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Vaping-linked lung injury: 2,172 cases, 42 deaths
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
REPORTING FROM CDC
Environmental Scan: Drivers of social, political and environmental change
We are living through an era of rapidly accelerated social, political, and environmental change. Spiraling costs of medical care, consumer activism around health care delivery, an aging population, and growing evidence of climate change are just some of the big currents of change. These trends are national and global in scope, and as such, far beyond any one profession or sector to shape or control. It remains for the medical profession to understand the currents of the time and adapt in order to thrive in the future.
Regulatory environment in flux
Hospitals and clinicians continue to struggle with a regulatory framework designed to improve higher quality of care yet may be creating additional barriers to access and efficiency. The passionate debate about health care costs and coverage is ongoing at the national level and appears to be a central issue on the minds of voters. Although the outcome of the debate cannot be foreseen, it will be left to the medical profession to maintain standards of care. Although the Affordable Care Act may not be repealed, the federal government’s role may diminish as policy is likely to be made by state politicians and bureaucrats.1 As a result, organizations operating in multiple states may find it difficult to develop organization-wide business strategies. And with the shift to value-based reimbursement and issues related to data breaches regarding private patient health care information, many health care professionals will need better support and documentation tools to remain compliant. This puts a large burden on medical organizations to invest even more in information technology, data management systems, and a wide range of training up and down the organizational chart. Keeping up with the needs of physicians for secure data management will be costly but critical.
Patients will feel climate change
Environmental factors affecting the air we breathe are of primary concern for patients with a broad range of cardiorespiratory conditions.2 Healthy but vulnerable infants, children, pregnant women, and the elderly may also feel the effects.3 Air pollution, increased levels of pollen and ground-level ozone, and wildfire smoke are all tied to climate change and all can have a direct impact on the patients seen by chest physicians.4 Individuals exposed to these environmental conditions may experience diminished lung function, resulting in increased hospital admissions.
Keeping up with the latest research on probable health impacts of these environmental trends will be on the agenda of most chest physicians.5 Professional societies will need to prepare to serve the educational needs of members in this regard. Continuing education content on these topics will be needed. The field will respond with new diagnostic tools and new treatments.6 Climate change may be a global-level phenomenon, but for many chest physicians, it means treating increasing numbers of patients affected by pulmonary disease.
Mind the generation gap
The population in the United States is primarily under the age of 65 years (84%), but the number of older citizens is on the rise. In 2016, there were 49.2 million people age 65 or older, and this number is projected to almost double to 98 million in 2060. The 85-and-over population is projected to more than double from 6.4 million in 2016 to 14.6 million in 2040 (a 129% increase).7
The medical needs of the aging population are already part of most medical institution’s planning but the current uncertainty in the health insurance market and the potential changes in Medicare coverage, not to mention the well-documented upcoming physician shortage, are complicating the planning process.8 Almost all acknowledge the “graying” of the population, but current approaches may not be sufficient given the projected the scale of the problems such as major increases in patients with chronic illnesses and the need for upscaling long-term geriatric care.
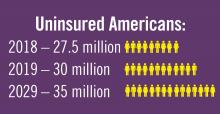
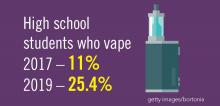
Planning for treating a growing elderly population shouldn’t mean ignoring some trends appearing among the younger population. E-cigarette use among middle- and high-school students may be creating millions of future patients with lung damage and nicotine addictions.9 Government intervention in this smoking epidemic is lagging behind the rapid spread of this unhealthy habit among young people.10 In 2019, health coverage of adults has begun to decline again after a decade of gains, and the possibility of this becoming a long-term trend has to be considered in planning for their treatment.11,12
References
1. Statistica. “Affordable Care Act - Statistics & Facts,” Matej Mikulic. 2019 Aug 14.
2. American Public Health Association and Centers for Disease Control and Prevention (2018). Climate Change Decreases the Quality of the Air We Breathe. Accessed 2019 Oct 7.
3. JAMA. 2019 Aug 13;322(6):546-56. doi: 10.1001/jama.2019.10255.
4. Lelieveld J et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40(20); 1590-6. doi: 10.1093/eurheartj/ehz135
5. European Respiratory Society. CME Online: Air pollution and respiratory health. 2018 Jun 21.
6. Environmental Protection Agency. “Particle Pollution and Your Patients’ Health,” Continuing Education for Particle Pollution Course. 2019 May 13.
7. U.S. Department of Health & Human Services. Administration on Aging. 2017 Profile of Older Americans. Accessed 2019 Oct 7.
8. Association of American Medical Colleges. Complexities of Physician Supply and Demand: Projection from 2017 to 2032. 2019 Apr.
9. Miech R et al. Trends in Adolescent Vaping, 2017-2019. N Eng J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739
10. Ned Sharpless, MD, Food and Drug Administration Acting Commissioner. “How the FDA is Regulating E-Cigarettes,” 2019 Sep 10.
11. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2019 to 2029. Washington D;C.: GPO, 2029
12. Galewitz P. “Breaking a 10-year streak, the number of uninsured Americans rises.” Internal Medicine News, 2019 Sep 11.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
We are living through an era of rapidly accelerated social, political, and environmental change. Spiraling costs of medical care, consumer activism around health care delivery, an aging population, and growing evidence of climate change are just some of the big currents of change. These trends are national and global in scope, and as such, far beyond any one profession or sector to shape or control. It remains for the medical profession to understand the currents of the time and adapt in order to thrive in the future.
Regulatory environment in flux
Hospitals and clinicians continue to struggle with a regulatory framework designed to improve higher quality of care yet may be creating additional barriers to access and efficiency. The passionate debate about health care costs and coverage is ongoing at the national level and appears to be a central issue on the minds of voters. Although the outcome of the debate cannot be foreseen, it will be left to the medical profession to maintain standards of care. Although the Affordable Care Act may not be repealed, the federal government’s role may diminish as policy is likely to be made by state politicians and bureaucrats.1 As a result, organizations operating in multiple states may find it difficult to develop organization-wide business strategies. And with the shift to value-based reimbursement and issues related to data breaches regarding private patient health care information, many health care professionals will need better support and documentation tools to remain compliant. This puts a large burden on medical organizations to invest even more in information technology, data management systems, and a wide range of training up and down the organizational chart. Keeping up with the needs of physicians for secure data management will be costly but critical.
Patients will feel climate change
Environmental factors affecting the air we breathe are of primary concern for patients with a broad range of cardiorespiratory conditions.2 Healthy but vulnerable infants, children, pregnant women, and the elderly may also feel the effects.3 Air pollution, increased levels of pollen and ground-level ozone, and wildfire smoke are all tied to climate change and all can have a direct impact on the patients seen by chest physicians.4 Individuals exposed to these environmental conditions may experience diminished lung function, resulting in increased hospital admissions.
Keeping up with the latest research on probable health impacts of these environmental trends will be on the agenda of most chest physicians.5 Professional societies will need to prepare to serve the educational needs of members in this regard. Continuing education content on these topics will be needed. The field will respond with new diagnostic tools and new treatments.6 Climate change may be a global-level phenomenon, but for many chest physicians, it means treating increasing numbers of patients affected by pulmonary disease.
Mind the generation gap
The population in the United States is primarily under the age of 65 years (84%), but the number of older citizens is on the rise. In 2016, there were 49.2 million people age 65 or older, and this number is projected to almost double to 98 million in 2060. The 85-and-over population is projected to more than double from 6.4 million in 2016 to 14.6 million in 2040 (a 129% increase).7
The medical needs of the aging population are already part of most medical institution’s planning but the current uncertainty in the health insurance market and the potential changes in Medicare coverage, not to mention the well-documented upcoming physician shortage, are complicating the planning process.8 Almost all acknowledge the “graying” of the population, but current approaches may not be sufficient given the projected the scale of the problems such as major increases in patients with chronic illnesses and the need for upscaling long-term geriatric care.
Planning for treating a growing elderly population shouldn’t mean ignoring some trends appearing among the younger population. E-cigarette use among middle- and high-school students may be creating millions of future patients with lung damage and nicotine addictions.9 Government intervention in this smoking epidemic is lagging behind the rapid spread of this unhealthy habit among young people.10 In 2019, health coverage of adults has begun to decline again after a decade of gains, and the possibility of this becoming a long-term trend has to be considered in planning for their treatment.11,12
References
1. Statistica. “Affordable Care Act - Statistics & Facts,” Matej Mikulic. 2019 Aug 14.
2. American Public Health Association and Centers for Disease Control and Prevention (2018). Climate Change Decreases the Quality of the Air We Breathe. Accessed 2019 Oct 7.
3. JAMA. 2019 Aug 13;322(6):546-56. doi: 10.1001/jama.2019.10255.
4. Lelieveld J et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40(20); 1590-6. doi: 10.1093/eurheartj/ehz135
5. European Respiratory Society. CME Online: Air pollution and respiratory health. 2018 Jun 21.
6. Environmental Protection Agency. “Particle Pollution and Your Patients’ Health,” Continuing Education for Particle Pollution Course. 2019 May 13.
7. U.S. Department of Health & Human Services. Administration on Aging. 2017 Profile of Older Americans. Accessed 2019 Oct 7.
8. Association of American Medical Colleges. Complexities of Physician Supply and Demand: Projection from 2017 to 2032. 2019 Apr.
9. Miech R et al. Trends in Adolescent Vaping, 2017-2019. N Eng J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739
10. Ned Sharpless, MD, Food and Drug Administration Acting Commissioner. “How the FDA is Regulating E-Cigarettes,” 2019 Sep 10.
11. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2019 to 2029. Washington D;C.: GPO, 2029
12. Galewitz P. “Breaking a 10-year streak, the number of uninsured Americans rises.” Internal Medicine News, 2019 Sep 11.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
We are living through an era of rapidly accelerated social, political, and environmental change. Spiraling costs of medical care, consumer activism around health care delivery, an aging population, and growing evidence of climate change are just some of the big currents of change. These trends are national and global in scope, and as such, far beyond any one profession or sector to shape or control. It remains for the medical profession to understand the currents of the time and adapt in order to thrive in the future.
Regulatory environment in flux
Hospitals and clinicians continue to struggle with a regulatory framework designed to improve higher quality of care yet may be creating additional barriers to access and efficiency. The passionate debate about health care costs and coverage is ongoing at the national level and appears to be a central issue on the minds of voters. Although the outcome of the debate cannot be foreseen, it will be left to the medical profession to maintain standards of care. Although the Affordable Care Act may not be repealed, the federal government’s role may diminish as policy is likely to be made by state politicians and bureaucrats.1 As a result, organizations operating in multiple states may find it difficult to develop organization-wide business strategies. And with the shift to value-based reimbursement and issues related to data breaches regarding private patient health care information, many health care professionals will need better support and documentation tools to remain compliant. This puts a large burden on medical organizations to invest even more in information technology, data management systems, and a wide range of training up and down the organizational chart. Keeping up with the needs of physicians for secure data management will be costly but critical.
Patients will feel climate change
Environmental factors affecting the air we breathe are of primary concern for patients with a broad range of cardiorespiratory conditions.2 Healthy but vulnerable infants, children, pregnant women, and the elderly may also feel the effects.3 Air pollution, increased levels of pollen and ground-level ozone, and wildfire smoke are all tied to climate change and all can have a direct impact on the patients seen by chest physicians.4 Individuals exposed to these environmental conditions may experience diminished lung function, resulting in increased hospital admissions.
Keeping up with the latest research on probable health impacts of these environmental trends will be on the agenda of most chest physicians.5 Professional societies will need to prepare to serve the educational needs of members in this regard. Continuing education content on these topics will be needed. The field will respond with new diagnostic tools and new treatments.6 Climate change may be a global-level phenomenon, but for many chest physicians, it means treating increasing numbers of patients affected by pulmonary disease.
Mind the generation gap
The population in the United States is primarily under the age of 65 years (84%), but the number of older citizens is on the rise. In 2016, there were 49.2 million people age 65 or older, and this number is projected to almost double to 98 million in 2060. The 85-and-over population is projected to more than double from 6.4 million in 2016 to 14.6 million in 2040 (a 129% increase).7
The medical needs of the aging population are already part of most medical institution’s planning but the current uncertainty in the health insurance market and the potential changes in Medicare coverage, not to mention the well-documented upcoming physician shortage, are complicating the planning process.8 Almost all acknowledge the “graying” of the population, but current approaches may not be sufficient given the projected the scale of the problems such as major increases in patients with chronic illnesses and the need for upscaling long-term geriatric care.
Planning for treating a growing elderly population shouldn’t mean ignoring some trends appearing among the younger population. E-cigarette use among middle- and high-school students may be creating millions of future patients with lung damage and nicotine addictions.9 Government intervention in this smoking epidemic is lagging behind the rapid spread of this unhealthy habit among young people.10 In 2019, health coverage of adults has begun to decline again after a decade of gains, and the possibility of this becoming a long-term trend has to be considered in planning for their treatment.11,12
References
1. Statistica. “Affordable Care Act - Statistics & Facts,” Matej Mikulic. 2019 Aug 14.
2. American Public Health Association and Centers for Disease Control and Prevention (2018). Climate Change Decreases the Quality of the Air We Breathe. Accessed 2019 Oct 7.
3. JAMA. 2019 Aug 13;322(6):546-56. doi: 10.1001/jama.2019.10255.
4. Lelieveld J et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40(20); 1590-6. doi: 10.1093/eurheartj/ehz135
5. European Respiratory Society. CME Online: Air pollution and respiratory health. 2018 Jun 21.
6. Environmental Protection Agency. “Particle Pollution and Your Patients’ Health,” Continuing Education for Particle Pollution Course. 2019 May 13.
7. U.S. Department of Health & Human Services. Administration on Aging. 2017 Profile of Older Americans. Accessed 2019 Oct 7.
8. Association of American Medical Colleges. Complexities of Physician Supply and Demand: Projection from 2017 to 2032. 2019 Apr.
9. Miech R et al. Trends in Adolescent Vaping, 2017-2019. N Eng J Med. 2019 Sep 18. doi: 10.1056/NEJMc1910739
10. Ned Sharpless, MD, Food and Drug Administration Acting Commissioner. “How the FDA is Regulating E-Cigarettes,” 2019 Sep 10.
11. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2019 to 2029. Washington D;C.: GPO, 2029
12. Galewitz P. “Breaking a 10-year streak, the number of uninsured Americans rises.” Internal Medicine News, 2019 Sep 11.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
CDC identifies probable culprit in vaping lung injuries
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
Vaping-linked lung injury cases near 1,900
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.
Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
CDC, FDA in hot pursuit of source of vaping lung injuries
The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
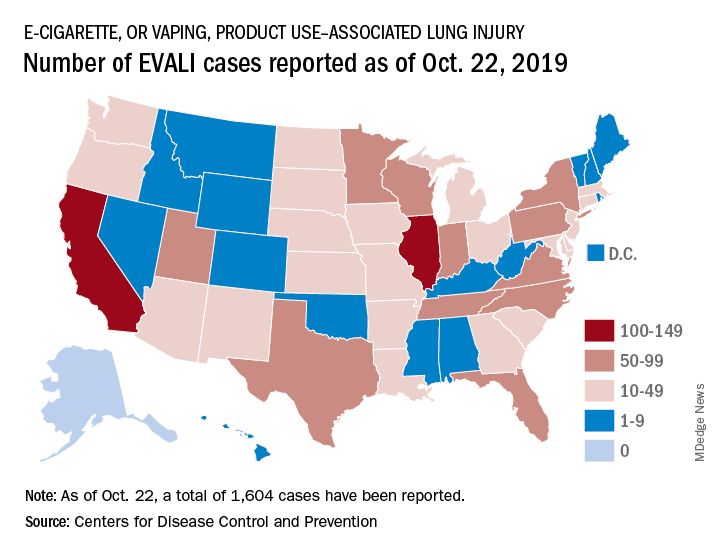
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
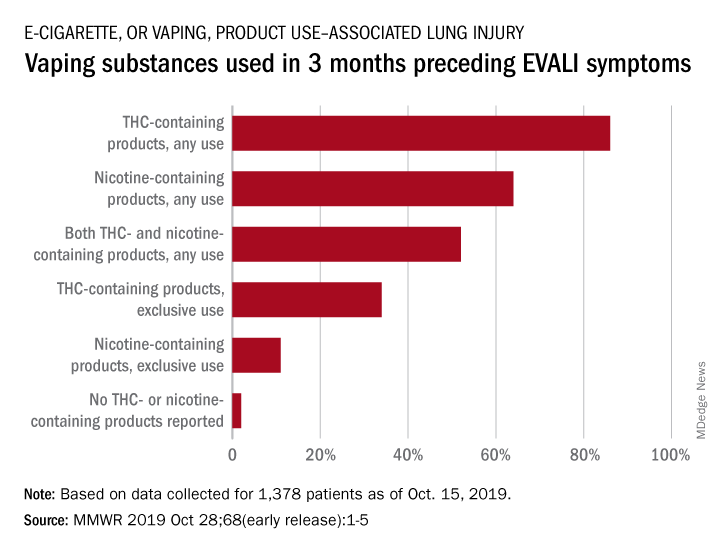
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.
Vaping-linked injuries top 1,600 cases
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
REPORTING FROM THE CDC
Dogs steal the show again at CHEST 2019
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
REPORTING FROM CHEST 2019
Vaping-linked lung injuries near 1,500
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
CDC updates guidance on vaping-associated lung injury
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
REPORTING FROM A CDC TELEBRIEFING