User login
The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
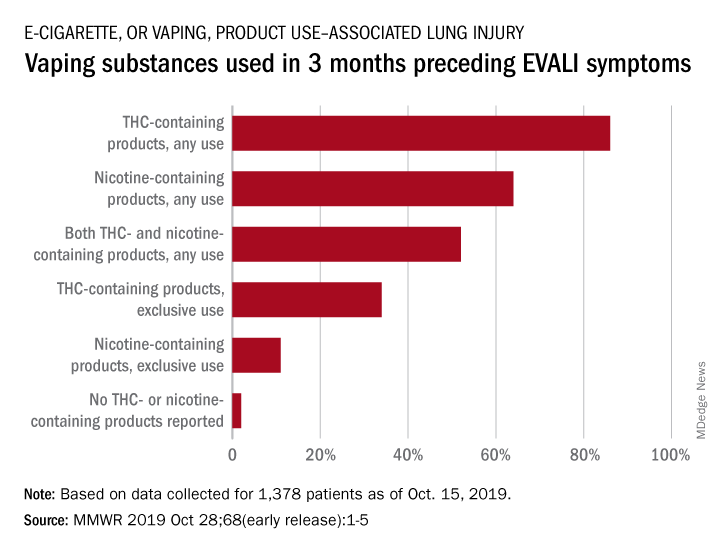
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.
