User login
Therese Borden is the editor of CHEST Physician. After 20 years of research, writing, and editing in the field of international development and economics, she began working in the field of medical editing and has held a variety of editorial positions with the company. She holds a PhD in International Economics from American University, Washington, and a BA in history from the University of Washington, Seattle.
On-site coverage of CHEST 2018
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
Refill disruptions for inhaled corticosteroids may mean more exacerbations
Interruptions of patients’ refills for combination inhaled corticosteroid medication caused by the Medicare Part D formulary switch may have resulted in increased exacerbations and hospitalizations, according to a study that will be presented at the CHEST 2018 annual meeting.
Katie Devane, PhD, and her colleagues examined pharmacy records of 44,832 patients aged 12 years and older who had received a combination inhaled corticosteroid (budesonide/formoterol) and a long-acting beta-agonist medication in 2016-2017. They were followed to track their refills, medication switches, and use of other medications such as oral corticosteroids, antibiotics, and rescue inhalers.
After the Medicare Part D formulary switch on Jan. 1, 2017, many of these patients experienced disruption of their refills. About half of the patients attempted to get a refill of their inhaled corticosteroid prescription but only 46% were approved. One-third of the patients studied did not replace their medication, 12% switched to monotherapy, and 17% had no inhaled medication, the study found.
The investigators concluded that the formulary block resulted in many patients going without optimal medication and potentially led to more exacerbations and ER visits.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31877-4/fulltext
The study will be presented in the session Improving Care in COPD, Monday, Oct. 8, 2:15 p.m., Convention Center Room 207A.
Interruptions of patients’ refills for combination inhaled corticosteroid medication caused by the Medicare Part D formulary switch may have resulted in increased exacerbations and hospitalizations, according to a study that will be presented at the CHEST 2018 annual meeting.
Katie Devane, PhD, and her colleagues examined pharmacy records of 44,832 patients aged 12 years and older who had received a combination inhaled corticosteroid (budesonide/formoterol) and a long-acting beta-agonist medication in 2016-2017. They were followed to track their refills, medication switches, and use of other medications such as oral corticosteroids, antibiotics, and rescue inhalers.
After the Medicare Part D formulary switch on Jan. 1, 2017, many of these patients experienced disruption of their refills. About half of the patients attempted to get a refill of their inhaled corticosteroid prescription but only 46% were approved. One-third of the patients studied did not replace their medication, 12% switched to monotherapy, and 17% had no inhaled medication, the study found.
The investigators concluded that the formulary block resulted in many patients going without optimal medication and potentially led to more exacerbations and ER visits.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31877-4/fulltext
The study will be presented in the session Improving Care in COPD, Monday, Oct. 8, 2:15 p.m., Convention Center Room 207A.
Interruptions of patients’ refills for combination inhaled corticosteroid medication caused by the Medicare Part D formulary switch may have resulted in increased exacerbations and hospitalizations, according to a study that will be presented at the CHEST 2018 annual meeting.
Katie Devane, PhD, and her colleagues examined pharmacy records of 44,832 patients aged 12 years and older who had received a combination inhaled corticosteroid (budesonide/formoterol) and a long-acting beta-agonist medication in 2016-2017. They were followed to track their refills, medication switches, and use of other medications such as oral corticosteroids, antibiotics, and rescue inhalers.
After the Medicare Part D formulary switch on Jan. 1, 2017, many of these patients experienced disruption of their refills. About half of the patients attempted to get a refill of their inhaled corticosteroid prescription but only 46% were approved. One-third of the patients studied did not replace their medication, 12% switched to monotherapy, and 17% had no inhaled medication, the study found.
The investigators concluded that the formulary block resulted in many patients going without optimal medication and potentially led to more exacerbations and ER visits.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31877-4/fulltext
The study will be presented in the session Improving Care in COPD, Monday, Oct. 8, 2:15 p.m., Convention Center Room 207A.
Treatment adherence may trump environmental factors for children with asthma
Children with asthma who are provided with care and medication per National Asthma Education and Prevention Program guidelines can improve over time, despite the presence of environmental factors such as second-hand tobacco smoke and domestic pets, according to a study presented at the CHEST 2018 annual meeting.
A study conducted at the Nationwide Children’s Hospital in Columbus, Ohio, included 395 children aged 2-17 years with a diagnosis of uncontrolled asthma. These children were then treated using the NAEPP guidelines for acute care needs and symptom control. In this sample of patients, 25% were exposed to second-hand smoke, and 55% had a cat or dog in the home.
The investigators followed these patients and observed improvement of symptoms. But in a comparison of those with and without the potentially problematic environmental factors, improvement was independent of the presence of these factors. The findings suggest that NAEPP-recommended treatment of asthma is more important than are some environmental factors.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31862-2/fulltext.
The findings will be presented in the session on Obstructive Lung Diseases, Wednesday, Oct. 10, at 1:00 p.m.
Children with asthma who are provided with care and medication per National Asthma Education and Prevention Program guidelines can improve over time, despite the presence of environmental factors such as second-hand tobacco smoke and domestic pets, according to a study presented at the CHEST 2018 annual meeting.
A study conducted at the Nationwide Children’s Hospital in Columbus, Ohio, included 395 children aged 2-17 years with a diagnosis of uncontrolled asthma. These children were then treated using the NAEPP guidelines for acute care needs and symptom control. In this sample of patients, 25% were exposed to second-hand smoke, and 55% had a cat or dog in the home.
The investigators followed these patients and observed improvement of symptoms. But in a comparison of those with and without the potentially problematic environmental factors, improvement was independent of the presence of these factors. The findings suggest that NAEPP-recommended treatment of asthma is more important than are some environmental factors.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31862-2/fulltext.
The findings will be presented in the session on Obstructive Lung Diseases, Wednesday, Oct. 10, at 1:00 p.m.
Children with asthma who are provided with care and medication per National Asthma Education and Prevention Program guidelines can improve over time, despite the presence of environmental factors such as second-hand tobacco smoke and domestic pets, according to a study presented at the CHEST 2018 annual meeting.
A study conducted at the Nationwide Children’s Hospital in Columbus, Ohio, included 395 children aged 2-17 years with a diagnosis of uncontrolled asthma. These children were then treated using the NAEPP guidelines for acute care needs and symptom control. In this sample of patients, 25% were exposed to second-hand smoke, and 55% had a cat or dog in the home.
The investigators followed these patients and observed improvement of symptoms. But in a comparison of those with and without the potentially problematic environmental factors, improvement was independent of the presence of these factors. The findings suggest that NAEPP-recommended treatment of asthma is more important than are some environmental factors.
View the study abstract here: https://journal.chestnet.org/article/S0012-3692(18)31862-2/fulltext.
The findings will be presented in the session on Obstructive Lung Diseases, Wednesday, Oct. 10, at 1:00 p.m.
Adherence to follow-up lung cancer screening not optimal
Former smokers’ adherence to annual follow-up screening for lung cancer was found to be less than optimal, according to a study to be presented at the CHEST 2018 annual meeting.
Paul B. Brasher, MD, and his colleagues from the Thoracic Oncology Research Group at the Medical University of South Carolina in Charleston studied adherence to recommended low-dose computed tomography (LDCT) among Veterans Affairs patients who were at high risk for lung cancer and whose baseline LDCTs were negative.
A total of 2,106 veterans aged 55-80 years who had at least a 30-pack year smoking history were initially screened within the Veterans Health Administration Lung Cancer Screening Demonstration Project. The study tracked 1,120 of these patients for 18 months to determine their adherence to annual LDCT screening; the rate of adherence was 77.6%.
View the abstract here: https://journal.chestnet.org/article/S0012-3692(18)31772-0/fulltext
The study will be presented in the session Lung Cancer Screening: New Questions and New Answers, Tuesday, Oct. 9, 8:45 a.m., Convention Center 207A.
Former smokers’ adherence to annual follow-up screening for lung cancer was found to be less than optimal, according to a study to be presented at the CHEST 2018 annual meeting.
Paul B. Brasher, MD, and his colleagues from the Thoracic Oncology Research Group at the Medical University of South Carolina in Charleston studied adherence to recommended low-dose computed tomography (LDCT) among Veterans Affairs patients who were at high risk for lung cancer and whose baseline LDCTs were negative.
A total of 2,106 veterans aged 55-80 years who had at least a 30-pack year smoking history were initially screened within the Veterans Health Administration Lung Cancer Screening Demonstration Project. The study tracked 1,120 of these patients for 18 months to determine their adherence to annual LDCT screening; the rate of adherence was 77.6%.
View the abstract here: https://journal.chestnet.org/article/S0012-3692(18)31772-0/fulltext
The study will be presented in the session Lung Cancer Screening: New Questions and New Answers, Tuesday, Oct. 9, 8:45 a.m., Convention Center 207A.
Former smokers’ adherence to annual follow-up screening for lung cancer was found to be less than optimal, according to a study to be presented at the CHEST 2018 annual meeting.
Paul B. Brasher, MD, and his colleagues from the Thoracic Oncology Research Group at the Medical University of South Carolina in Charleston studied adherence to recommended low-dose computed tomography (LDCT) among Veterans Affairs patients who were at high risk for lung cancer and whose baseline LDCTs were negative.
A total of 2,106 veterans aged 55-80 years who had at least a 30-pack year smoking history were initially screened within the Veterans Health Administration Lung Cancer Screening Demonstration Project. The study tracked 1,120 of these patients for 18 months to determine their adherence to annual LDCT screening; the rate of adherence was 77.6%.
View the abstract here: https://journal.chestnet.org/article/S0012-3692(18)31772-0/fulltext
The study will be presented in the session Lung Cancer Screening: New Questions and New Answers, Tuesday, Oct. 9, 8:45 a.m., Convention Center 207A.
Hernia registries need uniform data collection
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
FROM HERNIA
Nearly one-quarter of presurgery patients already using opioids
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.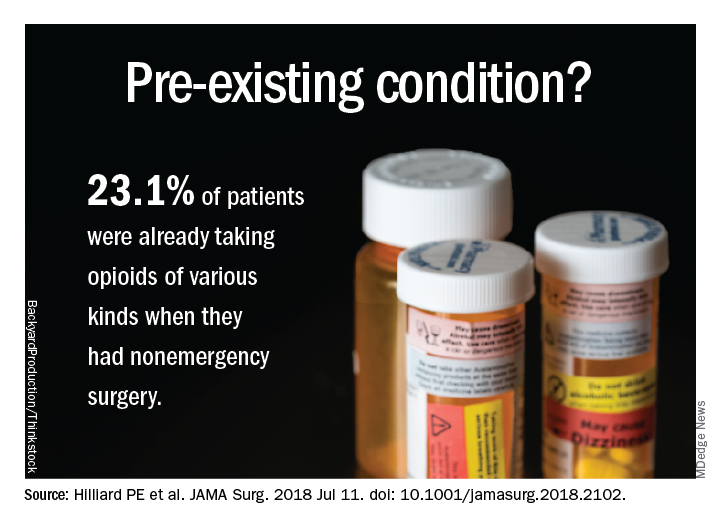
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.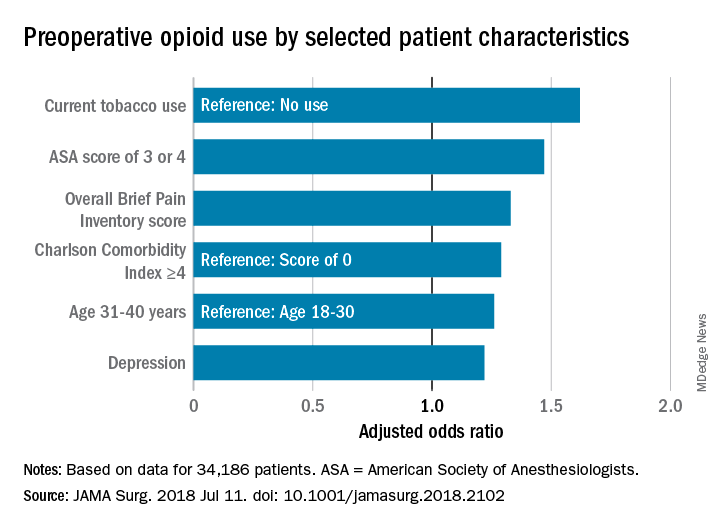
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
FROM JAMA SURGERY
Key clinical point: Preoperative opioid use is prevalent in patients who are having spinal surgery and have depression.
Major finding:
Study details: An observational study of 34,186 surgical patients in the University of Michigan Health system.
Disclosures: The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
Source: Hilliard P E et al. JAMA Surg. 2018 Jul 11;. doi:10.1001/jamasurg.2018.2102.
Bariatric revision mortality linked to age, comorbidities
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
REPORTING FROM DDW 2018
Key clinical point: Revisional bariatric procedures may carry a greater mortality risk than previous studies have suggested.
Major finding: The mortality rate in the sample was 2.1%.
Study details: The 2014 Nationwide Inpatient Sample database, comprising 14,280 patients who underwent revisional bariatric surgery.
Disclosures: Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
Source: Popov VB et al. DDW 2018, Abstract 324.
Sarcopenia had minor impact on hernia repair SSIs
according to findings from a prospective, single-institution study.
Steve R. Siegal, MD, and his colleagues at the Oregon Health & Science University, Portland, wrote in Hernia that risk factors for postoperative complications after hernia repair established in the literature include uncontrolled diabetes, active tobacco use, prior hernia repair, active infection, and obesity. Sarcopenia occurs in many physiological or pathological states, not only in the elderly but also in cases of immunosuppression, cirrhosis, trauma, prolonged immobility, and malignancy. Many opportunities exist for sarcopenia and hernia surgery to coincide but the role of sarcopenia in hernia repair outcomes has not been much studied.
The investigators began with the hypothesis that sarcopenia in hernia patients would lead to worse postoperative outcomes given the large metabolic requirement for postoperative healing of hernia defects and abdominal wall reconstruction.
The study involved 135 patients who underwent ventral hernia repair, 27% of whom had sarcopenia. The literature-based definition of sarcopenia was a muscle index cutoff of less than 52.4 cm2/m2 for men and less than 38.5 cm2/m2 for women. The investigators noted that the index cutoff was validated in oncology patients. Patients underwent a preoperative CT scan to assess muscle mass. The study group included patients with ventral hernia repair with or without mesh, component separation/abdominal wall reconstruction, and a hernia defect of 2 cm or larger.
With data on variables including gender, diabetes status, body mass index (BMI), chronic obstructive pulmonary disease (COPD), wound class, alcohol abuse status, and prior wound infections, the investigators created a multivariate model to look at primary outcomes of surgical site infection (SSI), surgical site occurrences (wound complications, other infections) and hernia recurrence. Secondary outcomes included length of hospital stay, morbidities, and other postoperative complications.
Patients with sarcopenia were more likely to have lower BMI (median 29.6 kg/m2 vs. 36.6 kg/m2), to be slightly older (median 63.1 vs. 59.2 years), and to have a history of immunosuppression (29.7% vs. 11.2%). The differences for the other variables (diabetes, tobacco use, COPD) were not significant.
The surprising finding was that sarcopenia was not significantly correlated with SSIs (P = 0.140), other complications (P = 0.113), or recurrence (P = 0.895) after ventral hernia repair. In-hospital morbidities and length of hospital stay did not differ significantly between the sarcopenic and nonsarcopenic patients. But sarcopenia combined with other factors did have an impact on outcomes. After adjustment for BMI and diabetes and critical care status, muscle mass as a continuous variable “was notable for a 1.44 increased odds [95% confidence interval, 1.00-2.07; P = 0.049] of inpatient morbidity with every decrease of 10 cm2/m2 of muscle index,” Dr. Siegal and his associates wrote.
The nonsignificant results of this study may be explained by the markedly lower BMI in the sarcopenic group, which could have been protective against worse outcomes, the investigators noted. However, they concluded, “Sarcopenia may still be a factor in adverse perioperative outcomes, but the established muscle index cutoff value may be inappropriately applied to benign patients, such as our ventral hernia cohort results in this study.”
The investigators declared no conflicts of interest and received no outside funding for this study.
SOURCE: Siegal SR et al. Hernia. 2018 May 11. doi: 10.1007/s10029-018-1770-8.
according to findings from a prospective, single-institution study.
Steve R. Siegal, MD, and his colleagues at the Oregon Health & Science University, Portland, wrote in Hernia that risk factors for postoperative complications after hernia repair established in the literature include uncontrolled diabetes, active tobacco use, prior hernia repair, active infection, and obesity. Sarcopenia occurs in many physiological or pathological states, not only in the elderly but also in cases of immunosuppression, cirrhosis, trauma, prolonged immobility, and malignancy. Many opportunities exist for sarcopenia and hernia surgery to coincide but the role of sarcopenia in hernia repair outcomes has not been much studied.
The investigators began with the hypothesis that sarcopenia in hernia patients would lead to worse postoperative outcomes given the large metabolic requirement for postoperative healing of hernia defects and abdominal wall reconstruction.
The study involved 135 patients who underwent ventral hernia repair, 27% of whom had sarcopenia. The literature-based definition of sarcopenia was a muscle index cutoff of less than 52.4 cm2/m2 for men and less than 38.5 cm2/m2 for women. The investigators noted that the index cutoff was validated in oncology patients. Patients underwent a preoperative CT scan to assess muscle mass. The study group included patients with ventral hernia repair with or without mesh, component separation/abdominal wall reconstruction, and a hernia defect of 2 cm or larger.
With data on variables including gender, diabetes status, body mass index (BMI), chronic obstructive pulmonary disease (COPD), wound class, alcohol abuse status, and prior wound infections, the investigators created a multivariate model to look at primary outcomes of surgical site infection (SSI), surgical site occurrences (wound complications, other infections) and hernia recurrence. Secondary outcomes included length of hospital stay, morbidities, and other postoperative complications.
Patients with sarcopenia were more likely to have lower BMI (median 29.6 kg/m2 vs. 36.6 kg/m2), to be slightly older (median 63.1 vs. 59.2 years), and to have a history of immunosuppression (29.7% vs. 11.2%). The differences for the other variables (diabetes, tobacco use, COPD) were not significant.
The surprising finding was that sarcopenia was not significantly correlated with SSIs (P = 0.140), other complications (P = 0.113), or recurrence (P = 0.895) after ventral hernia repair. In-hospital morbidities and length of hospital stay did not differ significantly between the sarcopenic and nonsarcopenic patients. But sarcopenia combined with other factors did have an impact on outcomes. After adjustment for BMI and diabetes and critical care status, muscle mass as a continuous variable “was notable for a 1.44 increased odds [95% confidence interval, 1.00-2.07; P = 0.049] of inpatient morbidity with every decrease of 10 cm2/m2 of muscle index,” Dr. Siegal and his associates wrote.
The nonsignificant results of this study may be explained by the markedly lower BMI in the sarcopenic group, which could have been protective against worse outcomes, the investigators noted. However, they concluded, “Sarcopenia may still be a factor in adverse perioperative outcomes, but the established muscle index cutoff value may be inappropriately applied to benign patients, such as our ventral hernia cohort results in this study.”
The investigators declared no conflicts of interest and received no outside funding for this study.
SOURCE: Siegal SR et al. Hernia. 2018 May 11. doi: 10.1007/s10029-018-1770-8.
according to findings from a prospective, single-institution study.
Steve R. Siegal, MD, and his colleagues at the Oregon Health & Science University, Portland, wrote in Hernia that risk factors for postoperative complications after hernia repair established in the literature include uncontrolled diabetes, active tobacco use, prior hernia repair, active infection, and obesity. Sarcopenia occurs in many physiological or pathological states, not only in the elderly but also in cases of immunosuppression, cirrhosis, trauma, prolonged immobility, and malignancy. Many opportunities exist for sarcopenia and hernia surgery to coincide but the role of sarcopenia in hernia repair outcomes has not been much studied.
The investigators began with the hypothesis that sarcopenia in hernia patients would lead to worse postoperative outcomes given the large metabolic requirement for postoperative healing of hernia defects and abdominal wall reconstruction.
The study involved 135 patients who underwent ventral hernia repair, 27% of whom had sarcopenia. The literature-based definition of sarcopenia was a muscle index cutoff of less than 52.4 cm2/m2 for men and less than 38.5 cm2/m2 for women. The investigators noted that the index cutoff was validated in oncology patients. Patients underwent a preoperative CT scan to assess muscle mass. The study group included patients with ventral hernia repair with or without mesh, component separation/abdominal wall reconstruction, and a hernia defect of 2 cm or larger.
With data on variables including gender, diabetes status, body mass index (BMI), chronic obstructive pulmonary disease (COPD), wound class, alcohol abuse status, and prior wound infections, the investigators created a multivariate model to look at primary outcomes of surgical site infection (SSI), surgical site occurrences (wound complications, other infections) and hernia recurrence. Secondary outcomes included length of hospital stay, morbidities, and other postoperative complications.
Patients with sarcopenia were more likely to have lower BMI (median 29.6 kg/m2 vs. 36.6 kg/m2), to be slightly older (median 63.1 vs. 59.2 years), and to have a history of immunosuppression (29.7% vs. 11.2%). The differences for the other variables (diabetes, tobacco use, COPD) were not significant.
The surprising finding was that sarcopenia was not significantly correlated with SSIs (P = 0.140), other complications (P = 0.113), or recurrence (P = 0.895) after ventral hernia repair. In-hospital morbidities and length of hospital stay did not differ significantly between the sarcopenic and nonsarcopenic patients. But sarcopenia combined with other factors did have an impact on outcomes. After adjustment for BMI and diabetes and critical care status, muscle mass as a continuous variable “was notable for a 1.44 increased odds [95% confidence interval, 1.00-2.07; P = 0.049] of inpatient morbidity with every decrease of 10 cm2/m2 of muscle index,” Dr. Siegal and his associates wrote.
The nonsignificant results of this study may be explained by the markedly lower BMI in the sarcopenic group, which could have been protective against worse outcomes, the investigators noted. However, they concluded, “Sarcopenia may still be a factor in adverse perioperative outcomes, but the established muscle index cutoff value may be inappropriately applied to benign patients, such as our ventral hernia cohort results in this study.”
The investigators declared no conflicts of interest and received no outside funding for this study.
SOURCE: Siegal SR et al. Hernia. 2018 May 11. doi: 10.1007/s10029-018-1770-8.
FROM HERNIA
Key clinical point: Sarcopenia was not correlated with surgical site infections, other complications, or recurrence after ventral hernia repair.
Major finding: After adjustment for body mass index and diabetes and critical care status, muscle mass as a continuous variable was notable for a 1.44 increased odds of inpatient morbidity.
Study details: A prospective, single-institution study of 135 patients who had ventral hernia repair, 27% of whom were sarcopenic.
Disclosures: The investigators declared no conflicts of interest and received no outside funding for this study.
Source: Siegal SR et al. Hernia. 2018 May 11. doi: 10.1007/s10029-018-1770-8.
Clinical Trial: Study looks at GI tract recovery after hernia repair
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
tborden@mdedge.com
SOURCE: Clinicaltrials.gov, NCT02379858.
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
tborden@mdedge.com
SOURCE: Clinicaltrials.gov, NCT02379858.
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
tborden@mdedge.com
SOURCE: Clinicaltrials.gov, NCT02379858.
FROM CLINICALTRIALS.COM
Clinical trial: Telescopic vs. balloon dissection for hernia
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
A randomized clinical trial will compare two techniques for achieving extraperitoneal space during hernia surgery.
The in operative times, early postoperative pain scores, surgical complications, and rate of hernia recurrence. The Spacemaker Balloon Dissector will be used for the trial.
The techniques will be timed and measured in minutes from incision to end of procedure. Pain outcomes will be measured using the Numeric Pain Rating Scale (NRS-11) at postoperative days 1, 7, and 30. Intraoperative complications, 30-day infections, and 1-year recurrence will be reported in numbers and percent as appropriate.
The study is sponsored by The Cleveland Clinic.
For more details about the trial, go to www.clinicaltrials.gov.
SOURCE: Clinical Trial NCT03276871.
FROM CLINICALTRIALS.GOV