User login
The Effect of Age on the Benefits of Early Decompression for Cervical Spondylotic Myelopathy
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
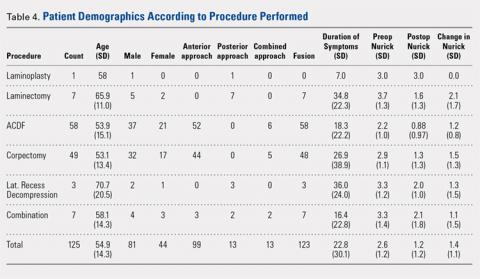
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
TAKE-HOME POINTS
- Decompression of cervical myelopathy within 24 months of symptom onset results in greater functional improvement compared to delayed decompression.
- The improvement with respect to time is more significant for patients older than 65 years compared to younger patients.
- Duration of symptoms does not seem to influence the severity of the preoperative Nurick score.
- Preoperative severity of symptoms is related to postoperative outcomes.
- Other significant predictors of worse outcomes include tobacco use, diabetes, and number of levels fused.