User login
Care of patients with dementia requires dynamic, creative approaches. Given the high incidence of sleep-wake disturbances in this population and the concomitant caregiver stress and institutionalization, researchers at the Geriatric Research Education and Clinical Center (GRECC) at the Louis Stokes Cleveland (LSC) VAMC in Ohio are exploring light as a nonpharmacologic intervention to improve sleep-wake activity in veterans with dementia.1
Light has a powerful physiologic effect on human circadian rhythms, and those who live in northern latitudes or spend long periods inside buildings often have limited exposure to dramatic light-dark differences required for circadian entrainment to the solar day. This article is intended to give a brief overview of the relationship between light and human health and describe initial pilot studies in improving rest-activity patterns through lighting.
Light and the Aging Eye
Light is received at the back of the eye and absorbed by retinal cells, rods, and cones and by intrinsically photosensitive retinal ganglion cells that are specialized circadian light receptors. These specialized receptors respond most strongly to short-wavelength (blue) light. Nerve pathways lead from the retina to the suprachiasmatic nucleus, the circadian pacemaker, influencing the secretion and suppression of biomarkers, such as melatonin, cortisol, and hypocretin.2 Many hormonal systems, including the steroid axis, key on this 24-hour cycle that seems to be partially timed via the hormone melatonin.
Serum melatonin signals darkness and nighttime to the body. It rises in the early evening, peaks in the middle of the night, and is low during the daytime hours. Light exposure at night can suppress melatonin, which can in turn alter the timing of circadian rhythm peaks and troughs.
Related: Development and Evaluation of a Geriatric Mood Management Program
Normal aging of the eye can include good acuity. Most older adults retain 20/25 or better vision in 1 eye into their 70s and 80s. However, changes in the anatomy of the eye due to aging can reduce contrast sensitivity, color vision, and visual fields. Reduction of light to the retina due to these normal physiologic changes can impair the response to circadian light. By the eighth and ninth decades of life, the amount of circadian activating light reaching the retina is one-tenth that for a 10 year old; thus, brighter white light or more short-wavelength light is needed.3
In addition to the anatomic changes in the eye, a muted 24-hour light exposure pattern contributes to circadian disruption in older adults. For those with dementia, the circadian disruption is manifested in rest-activity disorders, such as sundowning and day-night disorientation. Yet patients with dementia residing at home or in nursing homes routinely are exposed to subdued light patterns. For example, according to the results of a study, nursing home residents in San Diego, California, had a median of 10.5 min/d of exposure to bright light (> 1,000 lux) and median illuminance was 52 lux during daylight hours.4
How can light levels be changed to make them optimal or even sufficient for health? The researchers considered this question when opting to study light and its possible effects on older patients with dementia.
Lighting interventions in nursing homes may be effective. For example, 2 hours of bright light exposure in the morning improved sleep efficiency (number of minutes sleeping divided by number of minutes in bed).5 Furthermore, 30 minutes of sunlight per day has been shown to reduce daytime napping.6 In an investigation of long-term exposure to bright light, participants in the experimental group had less than the expected decline of their Mini-Mental State Examination scores across 3.5 years, improved depression scores, and less functional decline in their activities of daily living.7 Thus, lighting may have therapeutic effects for institutionalized patients with dementia, particularly if there is prolonged exposure.
Although increasing light levels improves rest-activity patterns in those with dementia, implementation and adherence is a challenge due to discomfort and glare, difficulty maintaining the level of light exposure due to a person’s activity, and/or energy codes that restrict power consumption in nursing homes. Although many human studies have used mixed spectrum light at high light levels, evidence suggests that the circadian system is maximally sensitive to shorter wavelength blue light.8 Therefore, short-wavelength blue light can achieve the same clinical outcomes while using lower illuminations and obviating many of the issues with bright lights.
Dayroom Transformation
In “light” of this background, the LSC VAMC GRECC engaged in a transformation of a community living center (CLC) dayroom to introduce circadian lighting, determine its acceptance to patients and staff, and measure rest-activity measurements for 3 residents. The CLC ward specializes in the care of veterans with dementia and had recently undergone a cultural transformation into a neighborhood system with many activities still centered on the dayroom/dining room. Based on the research of colleagues at the Lighting Research Center (LRC) at Rensselaer Polytechnic Institute (RPI) in Troy, New York, scientists at General Electric (GE) Lighting division in Cleveland developed fluorescent lamps emitting light in the short-wavelength portion of the visible spectrum (lamps with correlated color temperature [CCT] of 14,000 kelvin [K]; typical commercial use lamps have a CCT of 3,000-5,000 K).
Related: Home-Based Videotelehealth for Veterans With Dementia
The 14,000 K lamp, which was perceived as bluish-white or “blue sky” light, was chosen for installation. According to the model of human circadian phototransduction (the process in which the retina converts light signals into neural signals for the circadian system), the 14,000 K lamp can affect the circadian system at light levels much lower than those used in previously published studies (400-500 lux compared with > 2,500 lux).9 Changes in lamp spectrum and total irradiance emitted offered an 8-fold increase in circadian stimulation over the existing lighting. The LSC Human Subjects Review Board approved the project.
Measurements in the CLC ward indicated that existing lighting in the halls and rooms was dim—between 75 lux and 100 lux. Although this level was similar to those reported in the literature and satisfactory for reading and general activities, it likely was not sufficient to stimulate residents’ circadian systems.4 The dayroom was selected for lamp installation, because it was used for dining and many daily activities, thus maximizing the number of veterans who could benefit from exposure to the new type of lighting.
The dayroom was large, 35 feet by 40 feet, and had windows on 3 sides. Illumination came from the windows, which had blinds and/or window air conditioning units, and 13 ceiling fluorescent light fixtures, each with 4 lamps. Using multiple light meter measurements, 14,000 K lamps were installed in 7 of 13 light fixtures to minimize significant engineering changes while maximizing the illumination.
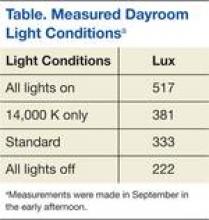
The Table shows the illumination in lux in the dayroom with and without the 14,000 K lighting. Horizontal light levels, 3 feet above the floor and measured in a horizontal direction, increased from between 300 lux and 350 lux to 500 lux. The lamps added more light and contrast while not increasing glare or causing excessive brightness.
Five veterans with dementia consented (with family members involved), but due to actigraph malfunction, only 3 of the 5 participants completed the 2 data collection periods: 7 continuous days of rest/activity measurements under the regular ward lighting and experimental lighting plan with a 3-week adaptation period in between. Results were generally in the expected direction after exposure to new lighting: Sleep latency (time in bed until the first 20 minutes of sleep) improved, decreasing by 23%, and sleep efficiency increased by 6.6%. The new lighting was well received; there were no reports about heat or glare, and the staff frequently commented that the room looked as though it was in reflected sunlight. A new CLC building was subsequently built with excellent window access and lighting. Therefore, the lighting project was moved to the home of a test subject.
Home Lighting Projects
In a feasibility pilot, the light exposure and rest activity of an older veteran with dementia and his spouse was measured in their home.10,11 Neither were exposed to light > 400 lux for much of the 7 continuous days of measurement, and the majority of their waking hours was spent in light < 100 lux (insufficient for reading). Actigraphy data indicated fragmented nighttime sleep for both participants with the caregiver sleeping much less than the veteran.
Related: New Guidelines on Concussion and Sleep Disturbance
This pilot suggested that appropriate circadian lighting in the home could positively influence circadian sleep-wake cycles. Therefore, in collaboration with colleagues at the LRC at RPI and GE, the authors initiated a study funded by the National Institute on Aging (PI: Figueiro M) to install home lighting customized to the rooms used most during the day by veterans with dementia. In phase 1, the results showed that circadian disruption in those with dementia in winter months was significantly higher than in the summer months and that healthy older adults received more circadian light and were less disrupted than those with dementia.12 Phase 2 of the study, which is a pretest/posttest control group intervention of circadian lighting, is ongoing.
Conclusion
The research has not yet provided a definitive answer about whether circadian-active light can improve circadian synchrony and thereby benefit patients with dementia and their caregivers. Work in this area is translational and ongoing: The issues of dosing, timing, and delivery are still open questions for further research. The next steps related to testing light delivery and dose could include tailoring the daytime lighting in day care centers with blue lighting and/or testing the use of blue light goggles.
Acknowledgements
The work discussed in this paper was funded in part by the Cleveland VISN 10 GRECC and the National Institute on Aging (grant # R01AG034157; Figueiro PI). GE Lighting, Cleveland, OH, USA donated the lamps used in the study.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(10):777-796.
2. Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12(1):188-200.
3. Turner PL, Mainster MA. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439-1444.
4. Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373-379.
5. Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly—an open trial. Int J Geriatr Psychiatry. 2003;18(6):520-526.
6. Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803-810.
7. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299(22):2642-2655.
8. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
9. Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50(2):213-228.
10. Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16(11):2292-2299.
11. Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: A methodological case study. Am J Alzheimers Dis Other Demen. 2010;25(4):353-361.
12. Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711-715.
Care of patients with dementia requires dynamic, creative approaches. Given the high incidence of sleep-wake disturbances in this population and the concomitant caregiver stress and institutionalization, researchers at the Geriatric Research Education and Clinical Center (GRECC) at the Louis Stokes Cleveland (LSC) VAMC in Ohio are exploring light as a nonpharmacologic intervention to improve sleep-wake activity in veterans with dementia.1
Light has a powerful physiologic effect on human circadian rhythms, and those who live in northern latitudes or spend long periods inside buildings often have limited exposure to dramatic light-dark differences required for circadian entrainment to the solar day. This article is intended to give a brief overview of the relationship between light and human health and describe initial pilot studies in improving rest-activity patterns through lighting.
Light and the Aging Eye
Light is received at the back of the eye and absorbed by retinal cells, rods, and cones and by intrinsically photosensitive retinal ganglion cells that are specialized circadian light receptors. These specialized receptors respond most strongly to short-wavelength (blue) light. Nerve pathways lead from the retina to the suprachiasmatic nucleus, the circadian pacemaker, influencing the secretion and suppression of biomarkers, such as melatonin, cortisol, and hypocretin.2 Many hormonal systems, including the steroid axis, key on this 24-hour cycle that seems to be partially timed via the hormone melatonin.
Serum melatonin signals darkness and nighttime to the body. It rises in the early evening, peaks in the middle of the night, and is low during the daytime hours. Light exposure at night can suppress melatonin, which can in turn alter the timing of circadian rhythm peaks and troughs.
Related: Development and Evaluation of a Geriatric Mood Management Program
Normal aging of the eye can include good acuity. Most older adults retain 20/25 or better vision in 1 eye into their 70s and 80s. However, changes in the anatomy of the eye due to aging can reduce contrast sensitivity, color vision, and visual fields. Reduction of light to the retina due to these normal physiologic changes can impair the response to circadian light. By the eighth and ninth decades of life, the amount of circadian activating light reaching the retina is one-tenth that for a 10 year old; thus, brighter white light or more short-wavelength light is needed.3
In addition to the anatomic changes in the eye, a muted 24-hour light exposure pattern contributes to circadian disruption in older adults. For those with dementia, the circadian disruption is manifested in rest-activity disorders, such as sundowning and day-night disorientation. Yet patients with dementia residing at home or in nursing homes routinely are exposed to subdued light patterns. For example, according to the results of a study, nursing home residents in San Diego, California, had a median of 10.5 min/d of exposure to bright light (> 1,000 lux) and median illuminance was 52 lux during daylight hours.4
How can light levels be changed to make them optimal or even sufficient for health? The researchers considered this question when opting to study light and its possible effects on older patients with dementia.
Lighting interventions in nursing homes may be effective. For example, 2 hours of bright light exposure in the morning improved sleep efficiency (number of minutes sleeping divided by number of minutes in bed).5 Furthermore, 30 minutes of sunlight per day has been shown to reduce daytime napping.6 In an investigation of long-term exposure to bright light, participants in the experimental group had less than the expected decline of their Mini-Mental State Examination scores across 3.5 years, improved depression scores, and less functional decline in their activities of daily living.7 Thus, lighting may have therapeutic effects for institutionalized patients with dementia, particularly if there is prolonged exposure.
Although increasing light levels improves rest-activity patterns in those with dementia, implementation and adherence is a challenge due to discomfort and glare, difficulty maintaining the level of light exposure due to a person’s activity, and/or energy codes that restrict power consumption in nursing homes. Although many human studies have used mixed spectrum light at high light levels, evidence suggests that the circadian system is maximally sensitive to shorter wavelength blue light.8 Therefore, short-wavelength blue light can achieve the same clinical outcomes while using lower illuminations and obviating many of the issues with bright lights.
Dayroom Transformation
In “light” of this background, the LSC VAMC GRECC engaged in a transformation of a community living center (CLC) dayroom to introduce circadian lighting, determine its acceptance to patients and staff, and measure rest-activity measurements for 3 residents. The CLC ward specializes in the care of veterans with dementia and had recently undergone a cultural transformation into a neighborhood system with many activities still centered on the dayroom/dining room. Based on the research of colleagues at the Lighting Research Center (LRC) at Rensselaer Polytechnic Institute (RPI) in Troy, New York, scientists at General Electric (GE) Lighting division in Cleveland developed fluorescent lamps emitting light in the short-wavelength portion of the visible spectrum (lamps with correlated color temperature [CCT] of 14,000 kelvin [K]; typical commercial use lamps have a CCT of 3,000-5,000 K).
Related: Home-Based Videotelehealth for Veterans With Dementia
The 14,000 K lamp, which was perceived as bluish-white or “blue sky” light, was chosen for installation. According to the model of human circadian phototransduction (the process in which the retina converts light signals into neural signals for the circadian system), the 14,000 K lamp can affect the circadian system at light levels much lower than those used in previously published studies (400-500 lux compared with > 2,500 lux).9 Changes in lamp spectrum and total irradiance emitted offered an 8-fold increase in circadian stimulation over the existing lighting. The LSC Human Subjects Review Board approved the project.
Measurements in the CLC ward indicated that existing lighting in the halls and rooms was dim—between 75 lux and 100 lux. Although this level was similar to those reported in the literature and satisfactory for reading and general activities, it likely was not sufficient to stimulate residents’ circadian systems.4 The dayroom was selected for lamp installation, because it was used for dining and many daily activities, thus maximizing the number of veterans who could benefit from exposure to the new type of lighting.
The dayroom was large, 35 feet by 40 feet, and had windows on 3 sides. Illumination came from the windows, which had blinds and/or window air conditioning units, and 13 ceiling fluorescent light fixtures, each with 4 lamps. Using multiple light meter measurements, 14,000 K lamps were installed in 7 of 13 light fixtures to minimize significant engineering changes while maximizing the illumination.
The Table shows the illumination in lux in the dayroom with and without the 14,000 K lighting. Horizontal light levels, 3 feet above the floor and measured in a horizontal direction, increased from between 300 lux and 350 lux to 500 lux. The lamps added more light and contrast while not increasing glare or causing excessive brightness.
Five veterans with dementia consented (with family members involved), but due to actigraph malfunction, only 3 of the 5 participants completed the 2 data collection periods: 7 continuous days of rest/activity measurements under the regular ward lighting and experimental lighting plan with a 3-week adaptation period in between. Results were generally in the expected direction after exposure to new lighting: Sleep latency (time in bed until the first 20 minutes of sleep) improved, decreasing by 23%, and sleep efficiency increased by 6.6%. The new lighting was well received; there were no reports about heat or glare, and the staff frequently commented that the room looked as though it was in reflected sunlight. A new CLC building was subsequently built with excellent window access and lighting. Therefore, the lighting project was moved to the home of a test subject.
Home Lighting Projects
In a feasibility pilot, the light exposure and rest activity of an older veteran with dementia and his spouse was measured in their home.10,11 Neither were exposed to light > 400 lux for much of the 7 continuous days of measurement, and the majority of their waking hours was spent in light < 100 lux (insufficient for reading). Actigraphy data indicated fragmented nighttime sleep for both participants with the caregiver sleeping much less than the veteran.
Related: New Guidelines on Concussion and Sleep Disturbance
This pilot suggested that appropriate circadian lighting in the home could positively influence circadian sleep-wake cycles. Therefore, in collaboration with colleagues at the LRC at RPI and GE, the authors initiated a study funded by the National Institute on Aging (PI: Figueiro M) to install home lighting customized to the rooms used most during the day by veterans with dementia. In phase 1, the results showed that circadian disruption in those with dementia in winter months was significantly higher than in the summer months and that healthy older adults received more circadian light and were less disrupted than those with dementia.12 Phase 2 of the study, which is a pretest/posttest control group intervention of circadian lighting, is ongoing.
Conclusion
The research has not yet provided a definitive answer about whether circadian-active light can improve circadian synchrony and thereby benefit patients with dementia and their caregivers. Work in this area is translational and ongoing: The issues of dosing, timing, and delivery are still open questions for further research. The next steps related to testing light delivery and dose could include tailoring the daytime lighting in day care centers with blue lighting and/or testing the use of blue light goggles.
Acknowledgements
The work discussed in this paper was funded in part by the Cleveland VISN 10 GRECC and the National Institute on Aging (grant # R01AG034157; Figueiro PI). GE Lighting, Cleveland, OH, USA donated the lamps used in the study.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Care of patients with dementia requires dynamic, creative approaches. Given the high incidence of sleep-wake disturbances in this population and the concomitant caregiver stress and institutionalization, researchers at the Geriatric Research Education and Clinical Center (GRECC) at the Louis Stokes Cleveland (LSC) VAMC in Ohio are exploring light as a nonpharmacologic intervention to improve sleep-wake activity in veterans with dementia.1
Light has a powerful physiologic effect on human circadian rhythms, and those who live in northern latitudes or spend long periods inside buildings often have limited exposure to dramatic light-dark differences required for circadian entrainment to the solar day. This article is intended to give a brief overview of the relationship between light and human health and describe initial pilot studies in improving rest-activity patterns through lighting.
Light and the Aging Eye
Light is received at the back of the eye and absorbed by retinal cells, rods, and cones and by intrinsically photosensitive retinal ganglion cells that are specialized circadian light receptors. These specialized receptors respond most strongly to short-wavelength (blue) light. Nerve pathways lead from the retina to the suprachiasmatic nucleus, the circadian pacemaker, influencing the secretion and suppression of biomarkers, such as melatonin, cortisol, and hypocretin.2 Many hormonal systems, including the steroid axis, key on this 24-hour cycle that seems to be partially timed via the hormone melatonin.
Serum melatonin signals darkness and nighttime to the body. It rises in the early evening, peaks in the middle of the night, and is low during the daytime hours. Light exposure at night can suppress melatonin, which can in turn alter the timing of circadian rhythm peaks and troughs.
Related: Development and Evaluation of a Geriatric Mood Management Program
Normal aging of the eye can include good acuity. Most older adults retain 20/25 or better vision in 1 eye into their 70s and 80s. However, changes in the anatomy of the eye due to aging can reduce contrast sensitivity, color vision, and visual fields. Reduction of light to the retina due to these normal physiologic changes can impair the response to circadian light. By the eighth and ninth decades of life, the amount of circadian activating light reaching the retina is one-tenth that for a 10 year old; thus, brighter white light or more short-wavelength light is needed.3
In addition to the anatomic changes in the eye, a muted 24-hour light exposure pattern contributes to circadian disruption in older adults. For those with dementia, the circadian disruption is manifested in rest-activity disorders, such as sundowning and day-night disorientation. Yet patients with dementia residing at home or in nursing homes routinely are exposed to subdued light patterns. For example, according to the results of a study, nursing home residents in San Diego, California, had a median of 10.5 min/d of exposure to bright light (> 1,000 lux) and median illuminance was 52 lux during daylight hours.4
How can light levels be changed to make them optimal or even sufficient for health? The researchers considered this question when opting to study light and its possible effects on older patients with dementia.
Lighting interventions in nursing homes may be effective. For example, 2 hours of bright light exposure in the morning improved sleep efficiency (number of minutes sleeping divided by number of minutes in bed).5 Furthermore, 30 minutes of sunlight per day has been shown to reduce daytime napping.6 In an investigation of long-term exposure to bright light, participants in the experimental group had less than the expected decline of their Mini-Mental State Examination scores across 3.5 years, improved depression scores, and less functional decline in their activities of daily living.7 Thus, lighting may have therapeutic effects for institutionalized patients with dementia, particularly if there is prolonged exposure.
Although increasing light levels improves rest-activity patterns in those with dementia, implementation and adherence is a challenge due to discomfort and glare, difficulty maintaining the level of light exposure due to a person’s activity, and/or energy codes that restrict power consumption in nursing homes. Although many human studies have used mixed spectrum light at high light levels, evidence suggests that the circadian system is maximally sensitive to shorter wavelength blue light.8 Therefore, short-wavelength blue light can achieve the same clinical outcomes while using lower illuminations and obviating many of the issues with bright lights.
Dayroom Transformation
In “light” of this background, the LSC VAMC GRECC engaged in a transformation of a community living center (CLC) dayroom to introduce circadian lighting, determine its acceptance to patients and staff, and measure rest-activity measurements for 3 residents. The CLC ward specializes in the care of veterans with dementia and had recently undergone a cultural transformation into a neighborhood system with many activities still centered on the dayroom/dining room. Based on the research of colleagues at the Lighting Research Center (LRC) at Rensselaer Polytechnic Institute (RPI) in Troy, New York, scientists at General Electric (GE) Lighting division in Cleveland developed fluorescent lamps emitting light in the short-wavelength portion of the visible spectrum (lamps with correlated color temperature [CCT] of 14,000 kelvin [K]; typical commercial use lamps have a CCT of 3,000-5,000 K).
Related: Home-Based Videotelehealth for Veterans With Dementia
The 14,000 K lamp, which was perceived as bluish-white or “blue sky” light, was chosen for installation. According to the model of human circadian phototransduction (the process in which the retina converts light signals into neural signals for the circadian system), the 14,000 K lamp can affect the circadian system at light levels much lower than those used in previously published studies (400-500 lux compared with > 2,500 lux).9 Changes in lamp spectrum and total irradiance emitted offered an 8-fold increase in circadian stimulation over the existing lighting. The LSC Human Subjects Review Board approved the project.
Measurements in the CLC ward indicated that existing lighting in the halls and rooms was dim—between 75 lux and 100 lux. Although this level was similar to those reported in the literature and satisfactory for reading and general activities, it likely was not sufficient to stimulate residents’ circadian systems.4 The dayroom was selected for lamp installation, because it was used for dining and many daily activities, thus maximizing the number of veterans who could benefit from exposure to the new type of lighting.
The dayroom was large, 35 feet by 40 feet, and had windows on 3 sides. Illumination came from the windows, which had blinds and/or window air conditioning units, and 13 ceiling fluorescent light fixtures, each with 4 lamps. Using multiple light meter measurements, 14,000 K lamps were installed in 7 of 13 light fixtures to minimize significant engineering changes while maximizing the illumination.
The Table shows the illumination in lux in the dayroom with and without the 14,000 K lighting. Horizontal light levels, 3 feet above the floor and measured in a horizontal direction, increased from between 300 lux and 350 lux to 500 lux. The lamps added more light and contrast while not increasing glare or causing excessive brightness.
Five veterans with dementia consented (with family members involved), but due to actigraph malfunction, only 3 of the 5 participants completed the 2 data collection periods: 7 continuous days of rest/activity measurements under the regular ward lighting and experimental lighting plan with a 3-week adaptation period in between. Results were generally in the expected direction after exposure to new lighting: Sleep latency (time in bed until the first 20 minutes of sleep) improved, decreasing by 23%, and sleep efficiency increased by 6.6%. The new lighting was well received; there were no reports about heat or glare, and the staff frequently commented that the room looked as though it was in reflected sunlight. A new CLC building was subsequently built with excellent window access and lighting. Therefore, the lighting project was moved to the home of a test subject.
Home Lighting Projects
In a feasibility pilot, the light exposure and rest activity of an older veteran with dementia and his spouse was measured in their home.10,11 Neither were exposed to light > 400 lux for much of the 7 continuous days of measurement, and the majority of their waking hours was spent in light < 100 lux (insufficient for reading). Actigraphy data indicated fragmented nighttime sleep for both participants with the caregiver sleeping much less than the veteran.
Related: New Guidelines on Concussion and Sleep Disturbance
This pilot suggested that appropriate circadian lighting in the home could positively influence circadian sleep-wake cycles. Therefore, in collaboration with colleagues at the LRC at RPI and GE, the authors initiated a study funded by the National Institute on Aging (PI: Figueiro M) to install home lighting customized to the rooms used most during the day by veterans with dementia. In phase 1, the results showed that circadian disruption in those with dementia in winter months was significantly higher than in the summer months and that healthy older adults received more circadian light and were less disrupted than those with dementia.12 Phase 2 of the study, which is a pretest/posttest control group intervention of circadian lighting, is ongoing.
Conclusion
The research has not yet provided a definitive answer about whether circadian-active light can improve circadian synchrony and thereby benefit patients with dementia and their caregivers. Work in this area is translational and ongoing: The issues of dosing, timing, and delivery are still open questions for further research. The next steps related to testing light delivery and dose could include tailoring the daytime lighting in day care centers with blue lighting and/or testing the use of blue light goggles.
Acknowledgements
The work discussed in this paper was funded in part by the Cleveland VISN 10 GRECC and the National Institute on Aging (grant # R01AG034157; Figueiro PI). GE Lighting, Cleveland, OH, USA donated the lamps used in the study.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(10):777-796.
2. Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12(1):188-200.
3. Turner PL, Mainster MA. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439-1444.
4. Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373-379.
5. Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly—an open trial. Int J Geriatr Psychiatry. 2003;18(6):520-526.
6. Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803-810.
7. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299(22):2642-2655.
8. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
9. Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50(2):213-228.
10. Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16(11):2292-2299.
11. Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: A methodological case study. Am J Alzheimers Dis Other Demen. 2010;25(4):353-361.
12. Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711-715.
1. Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(10):777-796.
2. Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12(1):188-200.
3. Turner PL, Mainster MA. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439-1444.
4. Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373-379.
5. Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly—an open trial. Int J Geriatr Psychiatry. 2003;18(6):520-526.
6. Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803-810.
7. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299(22):2642-2655.
8. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
9. Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50(2):213-228.
10. Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16(11):2292-2299.
11. Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: A methodological case study. Am J Alzheimers Dis Other Demen. 2010;25(4):353-361.
12. Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711-715.