User login
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
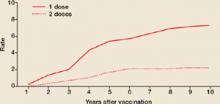
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.