User login
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
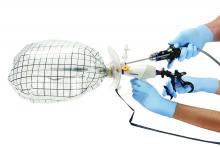
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
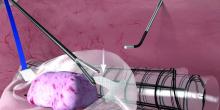
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
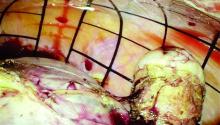
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.