Video

VIDEO: Tips for performing contained power morcellation
- Author:
- Tony Shibley, MD
Publish date: March 3, 2017
Dr. Tony Shibley offers his tips for performing power morcellation using the newly approved PneumoLiner containment device.
News
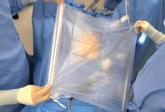
Safety techniques regarding morcellation
- Author:
- Tony Shibley, MD
- Bernard Taylor, MD
- Ceana H. Nezhat, MD
Publish date: June 16, 2014
