User login
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.
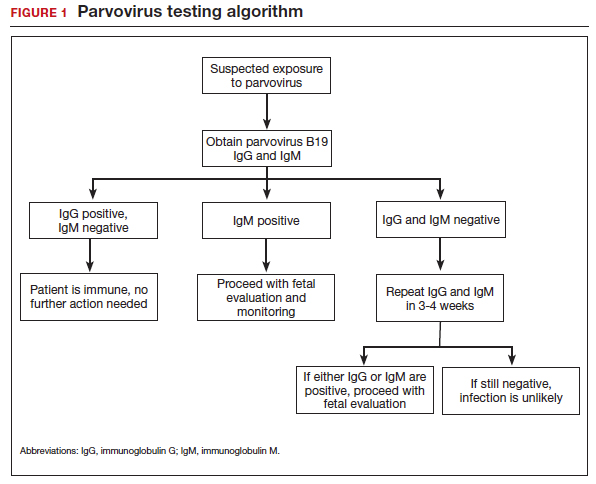
If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.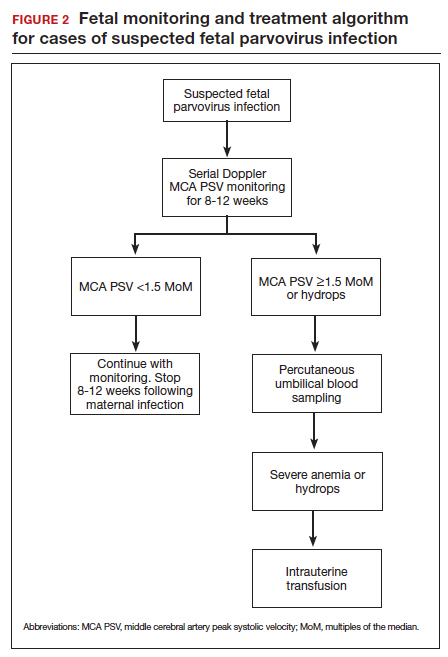
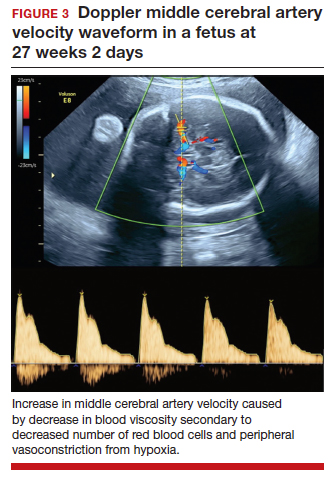
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.
Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.

If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.

Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.

If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.

Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.

